Regional Volume Decreases in the Brain of Pax6 Heterozygous Mutant Rats: MRI Deformation-Based Morphometry
- PMID: 27355350
- PMCID: PMC4927189
- DOI: 10.1371/journal.pone.0158153
Regional Volume Decreases in the Brain of Pax6 Heterozygous Mutant Rats: MRI Deformation-Based Morphometry
Abstract
Pax6 is a transcription factor that pleiotropically regulates various developmental processes in the central nervous system. In a previous study, we revealed that Pax6 heterozygous mutant (rSey2/+) adult rats exhibit abnormalities in social interaction. However, the brain malformations underlying the behavioral abnormality are unknown. To elucidate the brain malformations in rSey2/+ rats, we morphometrically analyzed brains of rSey2/+ and wild type rats using small-animal magnetic resonance imaging (MRI). Sixty 10-week-old rats underwent brain MRI (29 rSey2/+ rats and 31 wild type rats). SPM8 software was used for image preprocessing and statistical image analysis. Normalized maps of the Jacobian determinant, a parameter for the expansion and/or contraction of brain regions, were obtained for each rat. rSey2/+ rats showed significant volume decreases in various brain regions including the neocortex, corpus callosum, olfactory structures, hippocampal formation, diencephalon, and midbrain compared to wild type rats. Among brain regions, the anterior commissure showed significant interaction between genotype and sex, indicating the effect of genotype difference on the anterior commissure volume was more robust in females than in males. The rSey2/+ rats exhibited decreased volume in various gray and white matter regions of the brain, which may contribute to manifestation of abnormal social behaviors.
Conflict of interest statement
Figures
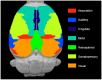
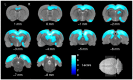



References
-
- Osumi N. The role of Pax6 in brain patterning. Tohoku J Exp Med. 2001;193:163–174. - PubMed
-
- Hever AM, Williamson KA, van Heyningen V. Developmental malformations of the eye: the role of PAX6, SOX2 and OTX2. Clin Genet. 2006;69:459–470. - PubMed
-
- Hanson IM. PAX6 and congenital eye malformations. Pediatr Res. 2003;54:791–796. - PubMed
-
- Ton CC, Hirvonen H, Miwa H, Weil MM, Monaghan P, Jordan T, et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059–1074. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

