Generation of Anti-Boa Immunoglobulin Antibodies for Serodiagnostic Applications, and Their Use to Detect Anti-Reptarenavirus Antibodies in Boa Constrictor
- PMID: 27355360
- PMCID: PMC4927170
- DOI: 10.1371/journal.pone.0158417
Generation of Anti-Boa Immunoglobulin Antibodies for Serodiagnostic Applications, and Their Use to Detect Anti-Reptarenavirus Antibodies in Boa Constrictor
Abstract
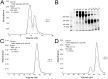
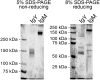
Immunoglobulins (Igs), the key effectors of the adaptive immune system, mediate the specific recognition of foreign structures, i.e. antigens. In mammals, IgM production commonly precedes the production of IgG in the response to an infection. The reptilian counterpart of IgG is IgY, but the exact kinetics of the reptilian immune response are less well known. Boid inclusion body disease (BIBD), an often fatal disease of captive boas and pythons has been linked to reptarenavirus infection, and BIBD is believed to be immunosuppressive. However, so far, the study of the serological response towards reptarenaviruses in BIBD has been hampered by the lack of reagents. Thus we set up a purification protocol for boa constrictor IgY and IgM, which should also be applicable for other snake species. We used centrifugal filter units, poly ethylene glycol precipitation and gel permeation chromatography to purify and separate the IgM and IgY fractions from boa constrictor serum, which we further used to immunise rabbits. We affinity purified IgM and IgY specific reagents from the produced antiserum, and labelled the reagents with horseradish peroxidase. Finally, using the sera of snakes with known exposure to reptarenaviruses we demonstrated that the newly generated reagents can be utilised for serodiagnostic purposes, such as immunoblotting and immunofluorescent staining. To our knowledge, this is the first report to show reptarenavirus-specific antibodies in boa constrictors.
Conflict of interest statement
Figures




References
-
- Litman GW, Anderson MK, Rast JP. Evolution of antigen binding receptors. Annu Rev Immunol. 1999. 17:109–147. - PubMed
-
- Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science. 1975. 188(4184):166–168. - PubMed
-
- Jaffredo T, Fellah JS, and Dunon D. Immunology of Birds and Reptiles. eLS. 2006. 10.1038/npg.els.0000521 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

