Synthesis of β-Branched Tryptophan Analogues Using an Engineered Subunit of Tryptophan Synthase
- PMID: 27355405
- PMCID: PMC4948191
- DOI: 10.1021/jacs.6b04836
Synthesis of β-Branched Tryptophan Analogues Using an Engineered Subunit of Tryptophan Synthase
Abstract
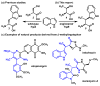
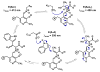
We report that l-threonine may substitute for l-serine in the β-substitution reaction of an engineered subunit of tryptophan synthase from Pyrococcus furiosus, yielding (2S,3S)-β-methyltryptophan (β-MeTrp) in a single step. The trace activity of the wild-type β-subunit on this substrate was enhanced more than 1000-fold by directed evolution. Structural and spectroscopic data indicate that this increase is correlated with stabilization of the electrophilic aminoacrylate intermediate. The engineered biocatalyst also reacts with a variety of indole analogues and thiophenol for diastereoselective C-C, C-N, and C-S bond-forming reactions. This new activity circumvents the 3-enzyme pathway that produces β-MeTrp in nature and offers a simple and expandable route to preparing derivatives of this valuable building block.
Figures


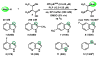
Similar articles
-
Directed evolution of the tryptophan synthase β-subunit for stand-alone function recapitulates allosteric activation.Proc Natl Acad Sci U S A. 2015 Nov 24;112(47):14599-604. doi: 10.1073/pnas.1516401112. Epub 2015 Nov 9. Proc Natl Acad Sci U S A. 2015. PMID: 26553994 Free PMC article.
-
Engineered Biosynthesis of β-Alkyl Tryptophan Analogues.Angew Chem Int Ed Engl. 2018 Nov 5;57(45):14764-14768. doi: 10.1002/anie.201807998. Epub 2018 Oct 12. Angew Chem Int Ed Engl. 2018. PMID: 30215880
-
Conformational Changes in the tryptophan synthase from a hyperthermophile upon alpha2beta2 complex formation: crystal structure of the complex.Biochemistry. 2005 Aug 30;44(34):11417-27. doi: 10.1021/bi050317h. Biochemistry. 2005. PMID: 16114878
-
Tryptophan Synthase: Biocatalyst Extraordinaire.Chembiochem. 2021 Jan 5;22(1):5-16. doi: 10.1002/cbic.202000379. Epub 2020 Sep 22. Chembiochem. 2021. PMID: 32677310 Free PMC article. Review.
-
Protein architecture, dynamics and allostery in tryptophan synthase channeling.Trends Biochem Sci. 1997 Jan;22(1):22-7. doi: 10.1016/s0968-0004(96)10066-9. Trends Biochem Sci. 1997. PMID: 9020588 Review.
Cited by
-
Biocatalysis: Enzymatic Synthesis for Industrial Applications.Angew Chem Int Ed Engl. 2021 Jan 4;60(1):88-119. doi: 10.1002/anie.202006648. Epub 2020 Aug 17. Angew Chem Int Ed Engl. 2021. PMID: 32558088 Free PMC article. Review.
-
Harnessing conformational dynamics in enzyme catalysis to achieve nature-like catalytic efficiencies: the shortest path map tool for computational enzyme redesign.Faraday Discuss. 2024 Sep 11;252(0):306-322. doi: 10.1039/d3fd00156c. Faraday Discuss. 2024. PMID: 38910409 Free PMC article.
-
Unlocking the function promiscuity of old yellow enzyme to catalyze asymmetric Morita-Baylis-Hillman reaction.Nat Commun. 2024 Jul 9;15(1):5737. doi: 10.1038/s41467-024-50141-2. Nat Commun. 2024. PMID: 38982157 Free PMC article.
-
Biocatalytic Friedel-Crafts Reactions.ChemCatChem. 2022 Sep 20;14(18):e202200636. doi: 10.1002/cctc.202200636. Epub 2022 Aug 24. ChemCatChem. 2022. PMID: 36606067 Free PMC article. Review.
-
Synthesis of tryptophan-containing 2,5-diketopiperazines via sequential C-H activation: total syntheses of tryprostatin A, maremycins A and B.Chem Sci. 2021 Sep 7;12(39):13137-13143. doi: 10.1039/d1sc02343h. eCollection 2021 Oct 13. Chem Sci. 2021. PMID: 34745544 Free PMC article.
References
-
- Alkhalaf LM, Ryan KS. Chem & Biol. 2015;22(3):317–328. - PubMed
-
- Wennemers H. Chem Comm. 2011;47(44):12036–1241. - PubMed
- Davie EAC, Mennen SM, Xu YJ, Miller SJ. Chem Rev. 2007;107(12):5759–5812. - PubMed
- Kiick KL, Saxon E, Tirrell DA, Bertozzi CR. Proc Natl Acad Sci USA. 2002;99(1):19–24. - PMC - PubMed
- Fowler VG, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, Levine DP, Chambers HF, Tally FP, Vigliani GA, Cabell CH, Link AS, DeMeyer I, Filler SG, Zervos M, Cook P, Parsonnet J, Bernstein JM, Price CS, Forrest GN, Fakenheuer G, Gareca M, Rehm SJ, Brodt HR, Tice A, Cosgrove SE. N Eng J of Med. 2006;355(7):653–665. - PubMed
-
- Sawai Y, Mizuno M, Ito T, Kawakami J, Yamano M. Tetrahedron. 2009;65(34):7122–7128.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

