DNA-catalyzed glycosylation using aryl glycoside donors
- PMID: 27355482
- PMCID: PMC4945402
- DOI: 10.1039/c6cc04329a
DNA-catalyzed glycosylation using aryl glycoside donors
Abstract
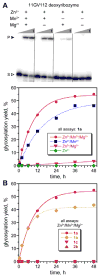
We report the identification by in vitro selection of Zn(2+)/Mn(2+)-dependent deoxyribozymes that glycosylate the 3'-OH of a DNA oligonucleotide. Both β and α anomers of aryl glycosides can be used as the glycosyl donors. Individual deoxyribozymes are each specific for a particular donor anomer.
Figures



References
-
- Schlosser K, Li Y. Chem Biol. 2009;16:311–322. - PubMed
- Silverman SK. Acc Chem Res. 2009;42:1521–1531. - PMC - PubMed
- Silverman SK. Angew Chem Int Ed. 2010;49:7180–7201. - PMC - PubMed
- Chandra M, Sachdeva A, Silverman SK. Nat Chem Biol. 2009;5:718–720. - PMC - PubMed
- Parker DJ, Xiao Y, Aguilar JM, Silverman SK. J Am Chem Soc. 2013;135:8472–8475. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

