Frequent Use of the IgA Isotype in Human B Cells Encoding Potent Norovirus-Specific Monoclonal Antibodies That Block HBGA Binding
- PMID: 27355511
- PMCID: PMC4927092
- DOI: 10.1371/journal.ppat.1005719
Frequent Use of the IgA Isotype in Human B Cells Encoding Potent Norovirus-Specific Monoclonal Antibodies That Block HBGA Binding
Abstract
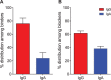
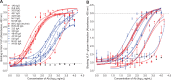
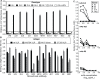
Noroviruses (NoV) are the most common cause of non-bacterial acute gastroenteritis and cause local outbreaks of illness, especially in confined situations. Despite being identified four decades ago, the correlates of protection against norovirus gastroenteritis are still being elucidated. Recent studies have shown an association of protection with NoV-specific serum histo-blood group antigen-blocking antibody and with serum IgA in patients vaccinated with NoV VLPs. Here, we describe the isolation and characterization of human monoclonal IgG and IgA antibodies against a GI.I NoV, Norwalk virus (NV). A higher proportion of the IgA antibodies blocked NV VLP binding to glycans than did IgG antibodies. We generated isotype-switched variants of IgG and IgA antibodies to study the effects of the constant domain on blocking and binding activities. The IgA form of antibodies appears to be more potent than the IgG form in blocking norovirus binding to histo-blood group antigens. These studies suggest a unique role for IgA antibodies in protection from NoV infections by blocking attachment to cell receptors.
Conflict of interest statement
MKE is named as an inventor on patents related to cloning of the Norwalk virus genome. She has an equity interest in Takeda Pharmaceuticals North America through LigoCyte and may potentially benefit from the research results. RLA has received research grant funding from and is a consultant to Takeda Vaccines, Inc. MKE, RLA, BVVP, GS and JEC, are named inventors on a submitted patent application that includes claims related to the antibodies in this paper.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

