Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children
- PMID: 27355532
- PMCID: PMC4962898
- DOI: 10.1056/NEJMoa1515257
Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children
Abstract
Background: The candidate malaria vaccine RTS,S/AS01 is being evaluated in order to inform a decision regarding its inclusion in routine vaccination schedules.
Methods: We conducted 7 years of follow-up in children who had been randomly assigned, at 5 to 17 months of age, to receive three doses of either the RTS,S/AS01 vaccine or a rabies (control) vaccine. The end point was clinical malaria (temperature of ≥37.5°C and infection with Plasmodium falciparum of >2500 parasites per cubic millimeter). In an analysis that was not prespecified, the malaria exposure of each child was estimated with the use of information on the prevalence of malaria among residents within a 1-km radius of the child's home. Vaccine efficacy was defined as 1 minus the hazard ratio or the incidence-rate ratio, multiplied by 100, in the RTS,S/AS01 group versus the control group.
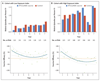
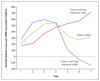
Results: Over 7 years of follow-up, we identified 1002 episodes of clinical malaria among 223 children randomly assigned to the RTS,S/AS01 group and 992 episodes among 224 children randomly assigned to the control group. The vaccine efficacy, as assessed by negative binomial regression, was 4.4% (95% confidence interval [CI], -17.0 to 21.9; P=0.66) in the intention-to-treat analysis and 7.0% (95% CI, -14.5 to 24.6; P=0.52) in the per-protocol analysis. Vaccine efficacy waned over time (P=0.006 for the interaction between vaccination and time), including negative efficacy during the fifth year among children with higher-than-average exposure to malaria parasites (intention-to-treat analysis: -43.5%; 95% CI, -100.3 to -2.8 [P=0.03]; per-protocol analysis: -56.8%; 95% CI, -118.7 to -12.3 [P=0.008]).
Conclusions: A three-dose vaccination with RTS,S/AS01 was initially protective against clinical malaria, but this result was offset by rebound in later years in areas with higher-than-average exposure to malaria parasites. (Funded by the PATH Malaria Vaccine Initiative and others; ClinicalTrials.gov number, NCT00872963.).
Figures
Comment in
-
Implementation of RTS,S/AS01 Malaria Vaccine--The Need for Further Evidence.N Engl J Med. 2016 Jun 30;374(26):2596-7. doi: 10.1056/NEJMe1606007. N Engl J Med. 2016. PMID: 27355540 No abstract available.
-
Vaccination against malaria: A dream too distant?Natl Med J India. 2016 Sep-Oct;29(5):282-283. Natl Med J India. 2016. PMID: 28098083 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
