Convergent Mechanistic Features between the Structurally Diverse N- and O-Methyltransferases: Glycine N-Methyltransferase and Catechol O-Methyltransferase
- PMID: 27355841
- PMCID: PMC5270642
- DOI: 10.1021/jacs.6b03462
Convergent Mechanistic Features between the Structurally Diverse N- and O-Methyltransferases: Glycine N-Methyltransferase and Catechol O-Methyltransferase
Abstract
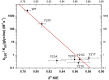
Although an enormous and still growing number of biologically diverse methyltransferases have been reported and identified, a comprehensive understanding of the enzymatic methyl transfer mechanism is still lacking. Glycine N-methyltransferase (GNMT), a member of the family that acts on small metabolites as the substrate, catalyzes methyl transfer from S-adenosyl-l-methionine (AdoMet) to glycine to form S-adenosyl-l-homocysteine and sarcosine. We report primary carbon ((12)C/(14)C) and secondary ((1)H3/(3)H3) kinetic isotope effects at the transferred methyl group, together with (1)H3/(3)H3 binding isotope effects for wild-type GNMT and a series of Tyr21 mutants. The data implicate a compaction effect in the methyl transfer step that is conferred by the protein structure. Furthermore, a remarkable similarity of properties is observed between GNMT and catechol O-methyltransferase, despite significant differences between these enzymes with regard to their active site structures and catalyzed reactions. We attribute these results to a catalytically relevant reduction in the methyl donor-acceptor distance that is dependent on a tyrosine side chain positioned behind the methyl-bearing sulfur of AdoMet.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
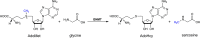



Similar articles
-
Mechanisms for auto-inhibition and forced product release in glycine N-methyltransferase: crystal structures of wild-type, mutant R175K and S-adenosylhomocysteine-bound R175K enzymes.J Mol Biol. 2000 Apr 21;298(1):149-62. doi: 10.1006/jmbi.2000.3637. J Mol Biol. 2000. PMID: 10756111
-
Structural Analysis of Glycine Sarcosine N-methyltransferase from Methanohalophilus portucalensis Reveals Mechanistic Insights into the Regulation of Methyltransferase Activity.Sci Rep. 2016 Dec 9;6:38071. doi: 10.1038/srep38071. Sci Rep. 2016. PMID: 27934872 Free PMC article.
-
Crystal structure of glycine N-methyltransferase from rat liver.Biochemistry. 1996 Sep 17;35(37):11985-93. doi: 10.1021/bi961068n. Biochemistry. 1996. PMID: 8810903
-
Structure and function of DNA methyltransferases.Annu Rev Biophys Biomol Struct. 1995;24:293-318. doi: 10.1146/annurev.bb.24.060195.001453. Annu Rev Biophys Biomol Struct. 1995. PMID: 7663118 Review.
-
Glycine and aging: Evidence and mechanisms.Ageing Res Rev. 2023 Jun;87:101922. doi: 10.1016/j.arr.2023.101922. Epub 2023 Mar 31. Ageing Res Rev. 2023. PMID: 37004845 Review.
Cited by
-
Hydrogen deuterium exchange defines catalytically linked regions of protein flexibility in the catechol O-methyltransferase reaction.Proc Natl Acad Sci U S A. 2020 May 19;117(20):10797-10805. doi: 10.1073/pnas.1917219117. Epub 2020 May 5. Proc Natl Acad Sci U S A. 2020. PMID: 32371482 Free PMC article.
-
Structure-function studies of tetrahydroprotoberberine N-methyltransferase reveal the molecular basis of stereoselective substrate recognition.J Biol Chem. 2019 Oct 4;294(40):14482-14498. doi: 10.1074/jbc.RA119.009214. Epub 2019 Aug 7. J Biol Chem. 2019. PMID: 31395658 Free PMC article.
-
Methyl transfer in psilocybin biosynthesis.Nat Commun. 2024 Mar 28;15(1):2709. doi: 10.1038/s41467-024-46997-z. Nat Commun. 2024. PMID: 38548735 Free PMC article.
-
Target Class Profiling of Small-Molecule Methyltransferases.ACS Chem Biol. 2023 Apr 21;18(4):969-981. doi: 10.1021/acschembio.3c00124. Epub 2023 Mar 28. ACS Chem Biol. 2023. PMID: 36976909 Free PMC article.
-
Structural and Functional Studies of Pavine N-Methyltransferase from Thalictrum flavum Reveal Novel Insights into Substrate Recognition and Catalytic Mechanism.J Biol Chem. 2016 Nov 4;291(45):23403-23415. doi: 10.1074/jbc.M116.747261. Epub 2016 Aug 29. J Biol Chem. 2016. PMID: 27573242 Free PMC article.
References
-
- Bruice T. C.; Lightstone F. C. Acc. Chem. Res. 1999, 32, 127–136. 10.1021/ar960131y. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

