Role of Heme Pocket Water in Allosteric Regulation of Ligand Reactivity in Human Hemoglobin
- PMID: 27355904
- PMCID: PMC4978812
- DOI: 10.1021/acs.biochem.6b00081
Role of Heme Pocket Water in Allosteric Regulation of Ligand Reactivity in Human Hemoglobin
Abstract
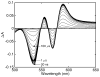
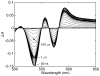
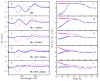
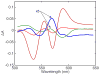
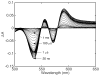
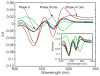
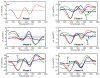
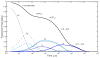
Water molecules can enter the heme pockets of unliganded myoglobins and hemoglobins, hydrogen bond with the distal histidine, and introduce steric barriers to ligand binding. The spectrokinetics of photodissociated CO complexes of human hemoglobin and its isolated α and β chains were analyzed for the effect of heme hydration on ligand rebinding. A strong coupling was observed between heme hydration and quaternary state. This coupling may contribute significantly to the 20-60-fold difference between the R- and T-state bimolecular CO binding rate constants and thus to the modulation of ligand reactivity that is the hallmark of hemoglobin allostery. Heme hydration proceeded over the course of several kinetic phases in the tetramer, including the R to T quaternary transition. An initial 150 ns hydration phase increased the R-state distal pocket water occupancy, nw(R), to a level similar to that of the isolated α (∼60%) and β (∼10%) chains, resulting in a modest barrier to ligand binding. A subsequent phase, concurrent with the first step of the R → T transition, further increased the level of heme hydration, increasing the barrier. The final phase, concurrent with the final step of the allosteric transition, brought the water occupancy of the T-state tetramer, nw(T), even higher and close to full occupancy in both the α and β subunits (∼90%). This hydration level could present an even larger barrier to ligand binding and contribute significantly to the lower iron reactivity of the T state toward CO.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Differential control of heme reactivity in alpha and beta subunits of hemoglobin: a combined Raman spectroscopic and computational study.J Am Chem Soc. 2014 Jul 23;136(29):10325-39. doi: 10.1021/ja503328a. Epub 2014 Jul 14. J Am Chem Soc. 2014. PMID: 24991732 Free PMC article.
-
The 1.9 A structure of deoxy beta 4 hemoglobin. Analysis of the partitioning of quaternary-associated and ligand-induced changes in tertiary structure.J Mol Biol. 1994 Feb 25;236(3):831-43. doi: 10.1006/jmbi.1994.1192. J Mol Biol. 1994. PMID: 8114097
-
Hemoglobin tertiary structural change on ligand binding. Its role in the co-operative mechanism.J Mol Biol. 1983 Dec 25;171(4):489-559. doi: 10.1016/0022-2836(83)90042-6. J Mol Biol. 1983. PMID: 6663623
-
Structural and thermodynamic aspects of cooperativity in the homodimeric hemoglobin from Scapharca inaequivalvis.Biophys Chem. 2000 Aug 30;86(2-3):173-8. doi: 10.1016/s0301-4622(00)00162-9. Biophys Chem. 2000. PMID: 11026682 Review.
-
Time-resolved optical spectroscopy and structural dynamics following photodissociation of carbonmonoxyhemoglobin.Biophys Chem. 1988 Feb;29(1-2):63-76. doi: 10.1016/0301-4622(88)87025-x. Biophys Chem. 1988. PMID: 3282562 Review.
Cited by
-
Dynamics of Quaternary Structure Transitions in R-State Carbonmonoxyhemoglobin Unveiled in Time-Resolved X-ray Scattering Patterns Following a Temperature Jump.J Phys Chem B. 2018 Dec 13;122(49):11488-11496. doi: 10.1021/acs.jpcb.8b07414. Epub 2018 Oct 16. J Phys Chem B. 2018. PMID: 30285440 Free PMC article.
-
Roles of Fe-Histidine bonds in stability of hemoglobin: Recognition of protein flexibility by Q Sepharose.Biophys J. 2021 Jul 6;120(13):2734-2745. doi: 10.1016/j.bpj.2021.05.014. Epub 2021 Jun 2. Biophys J. 2021. PMID: 34087219 Free PMC article.
-
Kinetic mechanisms for O2 binding to myoglobins and hemoglobins.Mol Aspects Med. 2022 Apr;84:101024. doi: 10.1016/j.mam.2021.101024. Epub 2021 Sep 17. Mol Aspects Med. 2022. PMID: 34544605 Free PMC article. Review.
References
-
- Quillin ML, Arduini RM, Olson JS, Phillips GN., Jr High-resolution crystal structures of distal histidine mutants of sperm whale myoglobin. J Mol Biol. 1993;234:140–155. - PubMed
-
- Olson JS, Phillips GN., Jr Myoglobin discriminates between O2, NO, and CO by electrostatic interactions with the bound ligand. JBIC, J Biol Inorg Chem. 1997;2:544–552.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

