Nutritional supplements for people being treated for active tuberculosis
- PMID: 27355911
- PMCID: PMC4981643
- DOI: 10.1002/14651858.CD006086.pub4
Nutritional supplements for people being treated for active tuberculosis
Abstract
Background: Tuberculosis and malnutrition are linked in a complex relationship. Tuberculosis may cause undernutrition through increased metabolic demands and decreased intake, and nutritional deficiencies may worsen the disease, or delay recovery by depressing important immune functions. At present, there is no evidence-based nutritional guidance for adults and children being treated for tuberculosis.
Objectives: To assess the effects of oral nutritional supplements in people being treated with antituberculous drug therapy for active tuberculosis.
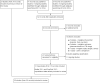
Search methods: We searched the Cochrane Infectious Disease Group Specialized Register, Cochrane Central Register of Controlled Trials (CENTRAL; Issue 1, 2016), MEDLINE (from 1946 to 4 February 2016), EMBASE (from 1980 to 4 February 2016), LILACS (from 1982 to 4 February 2016), the metaRegister of Controlled Trials (mRCT), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), and the Indian Journal of Tuberculosis up to 4 February 2016, and checked the reference lists of all included studies.
Selection criteria: Randomized controlled trials that compared any oral nutritional supplement given for at least four weeks with no nutritional intervention, placebo, or dietary advice only for people being treated for active tuberculosis. The primary outcomes of interest were all-cause death, and cure at six and 12 months.
Data collection and analysis: Two review authors independently selected trials for inclusion, and extracted data and assessed the risk of bias in the included trials. We presented the results as risk ratios (RR) for dichotomous variables, and mean differences (MD) for continuous variables, with 95% confidence intervals (CIs). Where appropriate, we pooled data from trials with similar interventions and outcomes. We assessed the quality of the evidence using the Grading of Recommendation Assessment, Development and Evaluation (GRADE) approach.
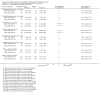
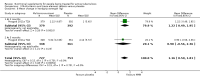
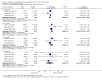
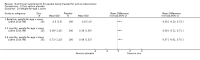
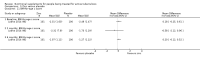
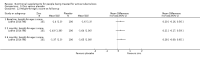
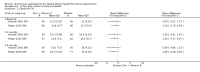
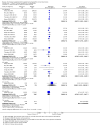
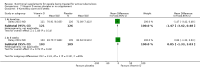
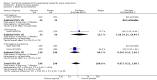
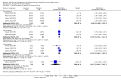
Main results: Thirty-five trials, including 8283 participants, met the inclusion criteria of this review. Macronutrient supplementationSix trials assessed the provision of free food, or high-energy supplements. Only two trials measured total dietary intake, and in both trials the intervention increased calorie consumption compared to controls.The available trials were too small to reliably prove or exclude clinically important benefits on mortality (RR 0.34, 95% CI 0.10 to 1.20; four trials, 567 participants, very low quality evidence), cure (RR 0.91, 95% CI 0.59 to 1.41; one trial, 102 participants, very low quality evidence), or treatment completion (data not pooled; two trials, 365 participants, very low quality evidence).Supplementation probably produces a modest increase in weight gain during treatment for active tuberculosis, although this was not seen consistently across all trials (data not pooled; five trials, 883 participants, moderate quality evidence). Two small studies provide some evidence that quality of life may also be improved but the trials were too small to have much confidence in the result (data not pooled; two trials, 134 participants, low quality evidence). Micronutrient supplementationSix trials assessed multi-micronutrient supplementation in doses up to 10 times the dietary reference intake, and 18 trials assessed single or dual micronutrient supplementation.Routine multi-micronutrient supplementation may have little or no effect on mortality in HIV-negative people with tuberculosis (RR 0.86, 95% CI 0.46 to 1.6; four trials, 1219 participants, low quality evidence), or HIV-positive people who are not taking antiretroviral therapy (RR 0.92, 95% CI 0.69 to 1.23; three trials, 1429 participants, moderate quality evidence). There is insufficient evidence to know if supplementation improves cure (no trials), treatment completion (RR 0.99, 95% CI 0.95 to 1.04; one trial, 302 participants, very low quality evidence), or the proportion of people who remain sputum positive during the first eight weeks (RR 0.92, 95% CI 0.63 to 1.35; two trials, 1020 participants, very low quality evidence). However, supplementation may have little or no effect on weight gain during treatment (data not pooled; five trials, 2940 participants, low quality evidence), and no studies have assessed the effect on quality of life.Plasma levels of vitamin A appear to increase following initiation of tuberculosis treatment regardless of supplementation. In contrast, supplementation probably does improve plasma levels of zinc, vitamin D, vitamin E, and selenium, but this has not been shown to have clinically important benefits. Of note, despite multiple studies of vitamin D supplementation in different doses, statistically significant benefits on sputum conversion have not been demonstrated.
Authors' conclusions: There is currently insufficient research to know whether routinely providing free food, or energy supplements improves tuberculosis treatment outcomes, but it probably improves weight gain in some settings.Although blood levels of some vitamins may be low in people starting treatment for active tuberculosis, there is currently no reliable evidence that routinely supplementing above recommended daily amounts has clinical benefits.
Conflict of interest statement
Liesl Grobler has no known conflicts of interest. David Sinclair was previously a member of the World Health Organization (WHO) Technical Advisory Group on Nutrition. This work may contribute to future recommendations on nutritional care in tuberculosis. Sukrti Nagpal has no known conflicts of interest. Thambu D Sudarsanam has no known conflicts of interest.
Figures






















































Update of
-
Nutritional supplements for people being treated for active tuberculosis.Cochrane Database Syst Rev. 2011 Nov 9;(11):CD006086. doi: 10.1002/14651858.CD006086.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2016 Jun 29;(6):CD006086. doi: 10.1002/14651858.CD006086.pub4. PMID: 22071828 Updated.
References
References to studies included in this review
Armijos 2010 MEX {published data only}
-
- Armijos RX, Weigel MM, Chacon R, Flores L, Campos A. Adjunctive micronutrient supplementation for pulmonary tuberculosis. Salud Pública de México 2010;52(3):185‐9. - PubMed
Daley 2015 IND {published data only}
-
- Daley P, Jagannathan V, John KR, Sarojini J, Latha A, Vieth R, et al. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double‐blind, placebo‐controlled trial. The Lancet. Infectious Diseases 2015;15(5):528‐34. - PubMed
Farazi 2015 IRN {published data only}
Ginawi 2013 IND {published data only}
-
- Ginawi IA, Ahmed MQ, Ahmad I, Al‐Hazimi AM. Effect of zinc and vitamin a supplementation along with inter‐tubercular treatment in pulmonary tuberculosis in north Indian patients. International Journal of Pharmaceutical Sciences and Research 2013;4(9):3426‐31.
Hanekom 1997 ZAF {published data only}
-
- Hanekom WA, Potgeiter S, Hughes EJ, Malan H, Kessow G, Hussey GD. Vitamin A status and therapy in childhood pulmonary tuberculosis. Journal of Pediatrics 1997;131(6):925‐7. - PubMed
Jahnavi 2010 IND {published data only}
-
- Jahnavi G, Sudha CH. Randomised controlled trial of food supplements in patients with newly diagnosed tuberculosis and wasting. Singapore Medical Journal 2010;51(12):957‐62. - PubMed
Jeremiah 2014 TZA {published data only}
Karyadi 2002 IDN {published data only}
-
- Karyadi E, West CE, Schultink W, Nelwan RH, Gross R, Amin Z, et al. A double‐blind, placebo‐controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: effects on clinical response and nutritional status. American Journal of Clinical Nutrition 2002;75(4):720‐7. - PubMed
Kota 2011 IND {published data only}
-
- Kota SK, Jammula S, Kota SK, Tripathy PR, Panda S, Modi KD. Effect of vitamin D supplementation in type 2 diabetes patients with pulmonary tuberculosis. Diabetes and Metabolic Syndrome: Clinical Research & Reviews 2011;5(2):85‐9. - PubMed
Lawson 2010 NGA {published data only}
-
- Lawson L, Thacher TD, Yassin MA, Onuoha NA, Usman A, Emenyonu NE, et al. Randomized controlled trial of zinc and vitamin A as co‐adjuvants for the treatment of pulmonary tuberculosis. Tropical Medicine and International Health 2010;15(12):1481‐90. - PubMed
Lodha 2014 IND {published data only}
-
- Lodha R, Mukherjee A, Singh V, Singh S, Friis H, Faurholt‐Jepsen D, et al. Effect of micronutrient supplementation on treatment outcomes in children with intrathoracic tuberculosis: a randomized controlled trial. American Journal of Clinical Nutrition 2014;100(5):1287‐97. - PubMed
Martineau 2011 GBR {published data only}
Martins 2009 TLS {published data only}
Mehta 2011 TZA {published and unpublished data}
-
- NCT00145184. Effect of Multivitamin Supplements on Clinical and Immunological Response in Childhood Tuberculosis. https://clinicaltrials.gov/ct2/show/NCT00145184 (accessed 4 February 2016).
Mily 2015 BGD {published data only}
Morcos 1998 EGY {published data only}
-
- Morcos MM, Gabr AA, Samual S, Kamel M, Baz M, Beshry M, et al. Vitamin D administration to tuberculous children and its value. Bollettino Chimico Farmaceutico 1998;137(5):157‐64. - PubMed
Nursyam 2006 IDN {published data only}
-
- Nursyam EW, Amin Z, Rumende CA. The effects of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculosis lesion. Acta Medica Indonesiana 2006;38(1):3‐5. - PubMed
Pakasi 2010 IDN {published data only}
Paliliewu 2013 IDN {published data only}
-
- Paliliewu N, Datau EA, Matheos JC, Surachmanto EE. Channa striatus capsules induces cytokine conversion in pulmonary tuberculosis patients. Journal of Experimental and Integrative Medicine 2013;3(3):237‐42.
Paton 2004 SGP {published data only}
-
- Paton NI, Chua YK, Earnest A, Chee CB. Randomized controlled trial of nutritional supplementation in patients with newly diagnosed tuberculosis and wasting. American Journal of Clinical Nutrition 2004;80(2):460‐5. - PubMed
Pérez‐Guzmán 2005 MEX {published data only}
-
- Pérez‐Guzmán C, Vargas MH, Quiñonez F, Bazavilvazo N, Aguilar A. A cholesterol‐rich diet accelerates bacteriologic sterilization in pulmonary tuberculosis. Chest 2005;127(2):643‐51. - PubMed
Praygod 2011a TZA {published data only}
-
- Praygod G, Range N, Faurholt‐Jepsen D, Jeremiah K, Faurholt‐Jepsen M, Aabye MG, et al. Daily multi‐micronutrient supplementation during tuberculosis treatment increases weight and grip strength among HIV‐uninfected but not HIV‐infected patients in Mwanza, Tanzania. The Journal of Nutrition 2011;141(4):685‐91. - PubMed
Praygod 2011b TZA {published data only}
-
- Praygod G, Range N, Faurholt‐Jepsen D, Jeremiah K, Faurholt‐Jepsen M, Aabye MG, et al. Daily multi‐micronutrient supplementation during tuberculosis treatment increases weight and grip strength among HIV‐uninfected but not HIV‐infected patients in Mwanza, Tanzania. The Journal of Nutrition 2011;141:685‐91. - PubMed
-
- Praygod G, Range N, Faurholt‐Jepsen D, Jeremiah K, Faurholt‐Jepsen M, Aabye MG, et al. The effect of energy‐protein supplementation on weight, body composition and handgrip strength among pulmonary tuberculosis HIV‐co‐infected patients: randomised controlled trial in Mwanza, Tanzania. The British Journal of Nutrition 2012;107(2):263‐71. - PubMed
Ralph 2013 IDN {published data only}
Range 2005 TZA {published data only}
-
- Range N, Andersen AB, Magnussen P, Mugomela A, Friis H. The effect of micronutrient supplementation on treatment outcome in patients with pulmonary tuberculosis: a randomised controlled trial in Mwanza, Tanzania. Tropical Medicine and International Health 2005;10(9):826‐32. - PubMed
-
- Range N, Changalucha J, Krarup H, Magnussen P, Andersen AB, Friis H. The effect of multi‐vitamin/mineral supplementation on mortality during treatment of pulmonary tuberculosis: a randomised two‐by‐two factorial trial in Mwanza, Tanzania. British Journal of Nutrition 2006;95(4):762‐70. - PubMed
Schön 2003 ETH {published data only}
-
- Schön T, Elias D, Moges F, Melese E, Tessema T, Stendahl O, et al. Arginine as an adjuvant to chemotherapy improves clinical outcomes in active tuberculosis. European Respiratory Journal 2003;21(3):483‐8. - PubMed
Schön 2011 ETH {published data only}
-
- Schön T, Idh J, Westman A, Elias D, Abate E, Diro E, et al. Effects of a food supplement rich in arginine in patients with smear positive pulmonary tuberculosis ‐ a randomised trial. Tuberculosis 2011;91(5):370‐7. - PubMed
Semba 2007 MWI {published data only}
-
- Semba RD, Kumwenda J, Zijlstra E, Ricks MO, Lettow M, Whalen C, et al. Micronutrient supplements and mortality of HIV‐infected adults with pulmonary TB: a controlled clinical trial. International Journal of Tuberculosis and Lung Disease 2007;11(8):854‐9. - PubMed
Seyedrezazadeh 2006 IRN {published data only}
-
- Seyedrezazadeh E, Ostadrahimi A, Mahboob S, Asadi Y, Ghaemmagami J, Pourmogaddam M. Effect of vitamin E and selenium supplementation on oxidative stress status in pulmonary tuberculosis patients. Respirology 2008;13(2):294‐8. - PubMed
-
- Seyedrezazadeh E, Ostradrahimi AR, Mahboob SA, Assadi Y, Ansarin K, Sakoori P, et al. Vitamin E‐selenium supplement and clinical responses of active pulmonary tuberculosis. Tanaffos 2006;5(2):49‐55.
Singh 2013 IND {published data only}
-
- Singh AKr, Gogoi JB, Pant NC, Mittal P, Juyal V, Mukherjee S. A study on the role of vitamins and minerals supplementation in the treatment of tuberculosis. Indian Journal of Public Health Research and Development 2013;4(2):26‐30.
Sudarsanam 2010 IND {published data only}
-
- Sudarsanam TD, John J, Kang G, Mahendri V, Gerrior J, Franciosa M, et al. Pilot randomized trial of nutritional supplementation in patients with tuberculosis and HIV‐tuberculosis coinfection receiving directly observed short‐course chemotherapy for tuberculosis. Tropical Medicine and International Health 2011;16(6):699‐706. - PMC - PubMed
Tukvadze 2015 GEO {published data only}
-
- Frediani J, Tukvadze N, Sanikidze E, Kipiani M, Hebbar G, Tangpricha V, et al. Drug susceptibility influences macronutrient intake and body composition in tuberculosis patients. The FASEB Journal 2014;28(Supplement 1):1014.4.
Villamor 2008 TZA {published and unpublished data}
-
- Villamor E, Mugusi F, Urassa W, Bosch R J, Saathoff E, Matsumoto K, et al. A trial of the effect of micronutrient supplementation on treatment outcome, T cell counts, morbidity, and mortality in adults with pulmonary tuberculosis. The Journal of Infectious Diseases 2008;197(11):1499‐505. - PMC - PubMed
Visser 2011 ZAF {published data only}
-
- Visser ME, Grewal HMS, Swart EC, Dhansay MA, Walzl G, Swanevelder S, et al. The effect of vitamin A and zinc supplementation on treatment outcomes in pulmonary tuberculosis: a randomized controlled trial. American Journal of Clinical Nutrition 2011;93(1):93‐100. - PubMed
Wejse 2008 GNB {published and unpublished data}
-
- Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, et al. Vitamin D as supplementary treatment for tuberculosis ‐ a double‐blind randomised placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2009;179(9):843‐50. - PubMed
References to studies excluded from this review
Denti 2015 {published data only}
Dibari 2013 {published data only}
-
- Dibari F, Bahwere P, Huerga H, Irena A H, Owino V, Collins S, et al. Development of a cross‐over randomized trial method to determine the acceptability and safety of novel ready‐to‐use therapeutic foods. Nutrition 2013;29(1):107‐12. - PubMed
Gwinup 1981 {published data only}
-
- Gwinup G, Randazzo G, Elias A. The influence of vitamin D on serum calcium in tuberculosis. Acta Endocrinologica 1981;97(1):114‐7. - PubMed
Hasan 2015 {published data only}
-
- Hasan H, Rini YP. The effect of propolis on the level of interleukin‐10 in multidrug resistance tuberculosis patient. Respirology 2015; Vol. 20, issue Suppl. 3:1‐160 (Abstract ID 869).
Kawai 2014 {published data only}
Khandelwal 2014 {published data only}
Lin 2014 {published data only}
-
- Lin HC, Yu MC, Liu HJ, Bai KJ. Impact of food intake on the pharmacokinetics of first‐line antituberculosis drugs in Taiwanese tuberculosis patients. Journal of the Formosan Medical Association 2014;113(5):291‐7. - PubMed
Lutge 2013 {published data only}
Martineau 2009 {published data only}
-
- Martineau AR, Nanzer AM, Satkunam KR, Packe GE, Rainbow SJ, Maunsell ZJ, et al. Influence of a single oral dose of vitamin D(2) on serum 25‐hydroxyvitamin D concentrations in tuberculosis patients. The International Journal of Tuberculosis and Lung Disease 2009;13(1):119‐25. - PubMed
Mbala 1998 {published data only}
-
- Mbala L, Matendo R, Nkailo R. Is vitamin B6 supplementation of isoniazid therapy useful in childhood tuberculosis. Tropical Doctor 1998;28(2):103‐4. - PubMed
Narang 1984 {published data only}
-
- Narang NK, Gupta RC, Jain MK. Role of vitamin D in pulmonary tuberculosis. Journal of the Association of Physicians of India 1984;32(2):185‐8. - PubMed
Oluboyede 1978 {published data only}
-
- Oluboyede OA, Onadeko BO. Observation on haematological patterns in pulmonary tuberculosis in Nigerians. Journal of Tropical Medicine and Hygiene 1978;81(5):91‐5. - PubMed
Permatasari 2014 {published data only}
-
- Permatasari A, Hasan H. The effect of ethanol extract propolis (EEP) on the level of IFN‐F and superoxide dismutase (SOD) activities in patients with MDR tuberculosis. Respirology 2014; Vol. 19, issue Suppl. 3:1‐62 (Abstract 0‐Q‐012).
Ramakrishnan 1961 {published data only}
Samsidi 2013 {published data only}
-
- Samsidi S, Ridwan H, Kusharto C M, Sulaeman A, Alisjahbana B. Efficacious of synbiotic and nutrients supplement on stimulated of secretory immunoglobulin a (SIGA) in treated pulmonary tuberculosis patients. Annals of Nutrition and Metabolism 2013;20th International Congress of Nutrition; Sept 15‐20 2013; Granada, Spain:407.
Shi 2001 {published data only}
-
- Shi XY, Xiao JQ, Yi JY, Zhu YQ, Deng CS. Influence of partial parenteral nutrition with fat emulsion on nutritional status in patients with abdominal tuberculosis. Journal of Clinical Internal Medicine 2001;18(2):120‐1.
Srivastava 2011 {published data only}
-
- Srivastava GN, Chaudhry S, Kumar H, Singh SK. Vitamin‐D supplementation in patients with new smear positive pulmonary tuberculosis (PTB) with reference to sputum conversion [Abstract]. European Respiratory Journal 2011;38(Supplement 55):2588.
References to studies awaiting assessment
Al Mamun 2014 {published data only}
-
- Al Mamun SMA. Effects and rationale of micronutrients supplementation in the treatment of smear positive pulmonary tuberculosis patients. Chest Journal 2014; Vol. 145, issue 3_Meeting Abstracts.
Chandra 2004 {published data only}
-
- Chandra RK. Nutrient supplementation as adjunct therapy in pulmonary tuberculosis. International Journal for Vitamin and Nutritional Research 2004;74(2):144‐6. - PubMed
Guzman‐Rivero 2013 {published data only}
-
- Guzman‐Rivero M, Sejas E, Medina M, Verduguez‐Orellana A, Akesson B. Changes in clinical status, nutritional and biochemical biomarkers in pulmonary tuberculosis patients receiving zinc supplementation in addition to conventional treatment (Abstract). Annals of Nutrition and Metabolism 2013;63(Supplement 1):1104.
Nagrale 2013 {published data only}
-
- Nagrale D, Mahakalkar S, Gaur S. Supplementation of N‐acetylcysteine as an adjuvant in treatment of newly diagnosed pulmonary tuberculosis patients: A prospective, randomized double blind, placebo controlled study (Abstract). European Respiratory Journal 2013;42(Suppl 57):P2833.
Nawas 2013 {published data only}
-
- Nawas MA, Burhan E, Soepandi P, Isbaniah F, Agustin H. Double blind randomized placebo‐controlled trial in tuberculosis patients with tuberculosis treatment supplemented with morrinda‐zinger extract: The speed of sputum conversion (Abstract). Respirology 2013;18(Supplement 4):9.
References to ongoing studies
ChiCTR‐IPR‐15006395 {unpublished data only}
-
- ChiCTR‐IPR‐15006395. The influence and mechanism of vitamin D3 supplementation on the treatment outcomes of tuberculosis patients of different glucose tolerance. http://www.chictr.org.cn/showproj.aspx?proj=10964 (accessed 4 February 2016).
ChiCTR‐TRC‐12002546 {published and unpublished data}
-
- Wang Q, Ma A, Bygbjerg IC, Han X, Liu Y, Zhao S, et al. Rationale and design of a randomized controlled trial of the effect of retinol and vitamin D supplementation on treatment in active pulmonary tuberculosis patients with diabetes. BMC Infectious Diseases 2013;13:104. [DOI: 10.1186/1471-2334-13-104] - DOI - PMC - PubMed
ChiCTR‐TRC‐14005241 {unpublished data only}
-
- ChiCTR‐TRC‐14005241. A prospective study of oral nutritional supplement in perioperative application with pulmonary tuberculosis patients. http://apps.who.int/trialsearch/Trial2.aspx?trialid=ChiCTR‐TRC‐14005241 (accessed 28 August 2015).
IRCT201112178429N1 {unpublished data only}
-
- IRCT201112178429N1. Effect of zinc supplementation in improving pulmonary tuberculosis patients in Qom. http://www.irct.ir/searchresult.php?keyword=&id=8429&number=1&am... (accessed 28 August 2015).
IRCT201211179855N2 {unpublished data only}
-
- IRCT201211179855N2. Randomized, double‐blind, placebo‐controlled trial of L‐Arginine supplementation for the treatment of pulmonary tuberculosis. http://www.irct.ir/searchresult.php?id=9855&number=2 (accessed 28 August 2015).
ISRCTN16469166 {unpublished data only}
-
- ISRCTN16469166. Nutrition and wasting in tuberculosis (TB): Can nutritional supplementation in TB patients improve body weight gain, body composition and treatment outcome?. www.controlled‐trials.com/ISRCTN16469166/ISRCTN16469166 (accessed 10 October 2007).
NCT00507000 {unpublished data only}
-
- NCT00507000. Role of oral vitamin D as an adjunct therapy in category I pulmonary tuberculosis along with assessment of immunological parameters. clinicaltrials.gov/ct2/show/NCT00507000 (accessed 10 October 2007).
NCT00698386 {unpublished data only}
-
- NCT00698386. Efficacy of oral zinc administration as an adjunct therapy in new pulmonary tuberculosis (Category I) patients. https://clinicaltrials.gov/ct2/show/NCT00698386 (accessed 21 July 2011).
NCT00788320 {unpublished data only}
-
- NCT00788320. Antimicrobial peptide LL‐37 (cathelicidin) production in active tuberculosis disease: role of vitamin D supplementation. https://clinicaltrials.gov/ct2/show/NCT00788320 (accessed 21 July 2011).
NCT01635153 {published data only}
-
- NCT01635153. Effects of a protein calorie supplement in HIV‐infected women with tuberculosis (DarDar). https://clinicaltrials.gov/ct2/show/NCT01635153 (accessed 31 August 2015).
NCT01657656 {published data only}
-
- NCT01657656. Vitamin D supplementations as adjunct to anti‐tuberculosis drugs in Mongolia. https://clinicaltrials.gov/ct2/show/record/NCT01657656 (accessed 31 August 2015).
NCT01722396 {published data only}
-
- NCT01722396. Pharmacogenetics of vitamin D supplementation in tuberculosis. https://clinicaltrials.gov/ct2/show/NCT01722396 (accessed 31 August 2015).
NCT01992263 {unpublished data only}
-
- NCT01992263. A trial of vitamin D supplementation among tuberculosis patients in South India. https://clinicaltrials.gov/ct2/show/NCT01992263 (accessed 31 August 2015).
NCT02169570 {unpublished data only}
-
- NCT02169570. Effect of supplementary vitamin D in patients with diabetes mellitus and pulmonary tuberculosis (EVIDENT). https://clinicaltrials.gov/ct2/show/NCT02169570 (accessed 31 August 2015).
NCT02464683 {unpublished data only}
-
- NCT02464683. Effect of vitamin D as adjunctive therapy in patients with pulmonary evolution tuberculosis (Vitamin D). https://clinicaltrials.gov/ct2/show/NCT02464683 (accessed 4 February 2016).
NCT02554318 {unpublished data only}
-
- NCT02554318. The effect of fermented soybean supplementation on the body weight and physical function of tuberculosis patients with standard therapy in Indonesia. https://clinicaltrials.gov/show/NCT02554318 (accessed 4 February 2016).
Additional references
Aaron 2004
-
- Aaron L, Saadoun D, Calatroni I, Launay O, Mémain N, Vincent V, et al. Tuberculosis in HIV‐infected patients: a comprehensive review. Clinical Microbiology and Infection 2004;10(5):388‐98. - PubMed
Arthur 2003
-
- Arthur JR, McKenziey RC, Beckett GJ. Selenium in the Immune System. The Journal of Nutrition 2003;133(5 Suppl 1):1457S‐9S. - PubMed
Barry 2009
Cegielski 2004
-
- Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis, evidence from studies in humans and experimental animals. International Journal of Tuberculosis and Lung Disease 2004;8(3):286‐98. - PubMed
Chandra 1996
-
- Chandra RK. Nutrition, immunity and infection: from basic knowledge of dietary manipulation of immune responses to practical application of ameliorating suffering and improving survival. Proceedings of the National Academy of Sciences of the United States of America 1996;93(25):14304‐7. - PMC - PubMed
Corbett 2003
-
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Archives of Internal Medicine 2003;163(9):1009‐21. - PubMed
Davies 1985
Grobler 2013
Harries 2006
-
- Harries AD, Dye C. Tuberculosis. Annals of Tropical Medicine and Parasitology 2006;100(5‐6):415‐31. - PubMed
Irlam 2010
Kamolratanakul 1999
-
- Kamolratanakul P, Sawert H, Kongsin S, Lertmaharit S, Sriwongsa J, Na‐Songkhla S, et al. Economic impact of tuberculosis at the household level. International Journal of Tuberculosis and Lung Disease 1999;3(7):596‐602. - PubMed
Lefebvre 2011
-
- Carol Lefebvre, Eric Manheimer, Julie Glanville, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://handbook.cochrane.org/chapter_6/6_4_11_1_the_cochrane_highly_sens....
Lounis 2001
-
- Lounis N, Truffot‐Pernot C, Grosset J, Gordeuk VR, Boelaert JR. Iron and Mycobacterium tuberculosis infection. Journal of Clinical Virology 2001;20(3):123‐6. - PubMed
Louw 1992
-
- Louw JA, Werbeck A, Louw MEJ, Kotze TJvW, Cooper R, Labadarios D. Blood vitamin concentrations during the acute phase response. Critical Care Medicine 1992;20(7):934‐41. - PubMed
Lutge 2009
-
- Lutge EE, Knight SE, Volmink J. Incentives for improving patient adherence to anti‐tuberculosis treatment. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007952] - DOI
Macallan 1999
-
- Macallan DC. Malnutrition in tuberculosis. Diagnostic Microbiology and Infectious Disease 1999;34(2):153‐7. - PubMed
Meyers 2006
-
- National Academy of Sciences. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. 6th Edition. Washington DC: National Academies Press, 2006.
nMaster 1.0 [Computer program]
-
- Department of Biostatistics, Christian Medical College. nMaster 1.0. Version 1.0. Vellore: Department of Biostatistics, Christian Medical College, (accessed 9 May 2016).
Nnoaham 2008
-
- Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta‐analysis. International Journal of Epidemiology 2008;37(1):113‐9. - PubMed
Plit 1998
-
- Plit Ml, Theron AJ, Fickl H, Rensburg CE, Pendel S, Anderson R. Influence of antimicrobial chemotherapy and smoking status on plasma concentrations of vitamin C, vitamin E, beta‐carotene, acute phase reactants, iron and lipid peroxides in patients with pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease 1998;2(7):590‐9. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saunders 2007
-
- Saunders BM, Britton WJ. Life and death in the granuloma: immunopathology of tuberculosis. Immunology and Cell Biology 2007;85(2):103‐11. - PubMed
Schluger 1998
-
- Schluger NW, Rom WN. The host immune response to tuberculosis. American Journal of Respiratory Critical Care Medicine 1998;157(3 Pt 1):679‐91. - PubMed
Semba 1998
-
- Semba RD. The role of vitamin A and related retinoids in immune function. Nutrition Reviews 1998;56(1 Pt 2):S38‐48. - PubMed
Shankar 1998
-
- Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. American Journal of Clinical Nutrition 1998;68(2 Suppl):447S‐63S. - PubMed
Stephensen 2001
-
- Stephensen CB. Vitamin A, infection, and immune function. Annual Review of Nutrition 2001;21:167‐92. - PubMed
Taneja 1990
-
- Taneja DP. Observations of serum zinc in patients with pulmonary tuberculosis. Journal of the Indian Medical Association 1990;88(10):280‐1. - PubMed
van Lettow 2003
-
- Lettow M, Fawzi WW, Semba RD. Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus coinfection. Nutrition Reviews 2003;61(3):81‐90. - PubMed
Wesje 2008
-
- Wesje C, Gustafson P, Nielson J, Gomes VF, Aaby P, Anderson PL, et al. TB score: Signs and symptoms from tuberculosis patients in a low‐resource setting have predictive value and may be used to assess clinical course. Scandinavian Journal of Infectious Diseases 2008;40(2):111‐20. - PubMed
WFP 2007
-
- Ba M, Strauss A. Getting started: WFP food assistance in the context of tuberculosis care and treatment. World Food Programme, 2007.
WHO 2009
-
- World Health Organization. Global Tuberculosis Control. A short update to the 2009 report. Geneva: World Health Organization, 2009.
WHO 2010
-
- World Health Organization. Treatment of Tuberculosis Guidelines. 4th Edition. Geneva: World Health Organization, 2010.
WHO 2014
-
- World Health Organization. Global Tuberculosis Report 2014. Geneva: World Health Organization, 2014.
Wintergerst 2007
-
- Wintergerst ES, Maggini S, Hornig DH. Contribution of Selected Vitamins andTrace Elements to Immune Function. Annals of Nutrition & Metabolism 2007;51(4):301‐23. - PubMed
Wyss 2001
-
- Wyss K, Kilima P, Lorenz N. Costs of tuberculosis for households and health care providers in Dar es Salaam, Tanzania. Tropical Medicine & International Health 2001;6(1):60‐8. - PubMed
Zachariah 2002
-
- Zachariah R, Spielmann MP, Harries AD, Salaniponi FM. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Transactions of the Royal Society of Tropical Medicine and Hygiene 2002;96(3):291‐4. - PubMed
References to other published versions of this review
Abba 2006
Abba 2008
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

