Spectroscopic and Kinetic Characterization of Peroxidase-Like π-Cation Radical Pinch-Porphyrin-Iron(III) Reaction Intermediate Models of Peroxidase Enzymes
- PMID: 27355940
- PMCID: PMC6273987
- DOI: 10.3390/molecules21070804
Spectroscopic and Kinetic Characterization of Peroxidase-Like π-Cation Radical Pinch-Porphyrin-Iron(III) Reaction Intermediate Models of Peroxidase Enzymes
Abstract
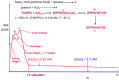
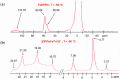
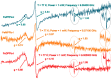
The spectroscopic and kinetic characterization of two intermediates from the H₂O₂ oxidation of three dimethyl ester [(proto), (meso), (deuteroporphyrinato) (picdien)]Fe(III) complexes ([FePPPic], [FeMPPic] and [FeDPPic], respectively) pinch-porphyrin peroxidase enzyme models, with s = 5/2 and 3/2 Fe(III) quantum mixed spin (qms) ground states is described herein. The kinetic study by UV/Vis at λmax = 465 nm showed two different types of kinetics during the oxidation process in the guaiacol test for peroxidases (1-3 + guaiacol + H₂O₂ → oxidation guaiacol products). The first intermediate was observed during the first 24 s of the reaction. When the reaction conditions were changed to higher concentration of pinch-porphyrins and hydrogen peroxide only one type of kinetics was observed. Next, the reaction was performed only between pinch-porphyrins-Fe(III) and H₂O₂, resulting in only two types of kinetics that were developed during the first 0-4 s. After this time a self-oxidation process was observed. Our hypotheses state that the formation of the π-cation radicals, reaction intermediates of the pinch-porphyrin-Fe(III) family with the ligand picdien [N,N'-bis-pyridin-2-ylmethyl-propane-1,3-diamine], occurred with unique kinetics that are different from the overall process and was involved in the oxidation pathway. UV-Vis, ¹H-NMR and ESR spectra confirmed the formation of such intermediates. The results in this paper highlight the link between different spectroscopic techniques that positively depict the kinetic traits of artificial compounds with enzyme-like activity.
Keywords: peroxidases models; pinch-porphyrins; spectroscopic studies; π-cation radical.
Conflict of interest statement
All author declare no conflict of interest.
Figures








Similar articles
-
New pinch-porphyrin complexes with quantum mixed spin ground state S=3/2,5/2 of iron (III) and their catalytic activity as peroxidase.Biophys Chem. 2003 Dec 1;106(3):253-65. doi: 10.1016/s0301-4622(03)00186-8. Biophys Chem. 2003. PMID: 14556897
-
Kinetic simulation studies on the transient formation of the oxo-iron(IV) porphyrin radical cation during the reaction of iron(III) tetrakis-5,10,15,20-(N-methyl-4-pyridyl)-porphyrin with hydrogen peroxide in aqueous solution.Luminescence. 2003 Sep-Oct;18(5):259-67. doi: 10.1002/bio.736. Luminescence. 2003. PMID: 14587077
-
Disproportionation of Iron(III) Porphyrin pi-Cation Radicals in the Presence of Sterically Hindered Pyridines. Spectroscopic Detection of Asymmetric Highly Oxidized Intermediates.Inorg Chem. 1996 Feb 28;35(5):1136-1147. doi: 10.1021/ic950876k. Inorg Chem. 1996. PMID: 11666301
-
Haem iron-containing peroxidases.Essays Biochem. 1999;34:51-69. doi: 10.1042/bse0340051. Essays Biochem. 1999. PMID: 10730188 Review.
-
Iron corrolates: unambiguous chloroiron(III) (corrolate)(2-.) pi-cation radicals.J Inorg Biochem. 2006 Apr;100(4):810-37. doi: 10.1016/j.jinorgbio.2006.01.038. Epub 2006 Mar 7. J Inorg Biochem. 2006. PMID: 16519943 Review.
Cited by
-
N-Confused Porphyrin Immobilized on Solid Supports: Synthesis and Metal Ions Sensing Efficacy.Molecules. 2018 Apr 10;23(4):867. doi: 10.3390/molecules23040867. Molecules. 2018. PMID: 29642601 Free PMC article.
-
Synthesis, Kinetic Study, and Spectroscopic Analysis of Peroxidase-like Pinch-Porphyrin Fe(III) Complexes.ACS Omega. 2019 Dec 19;4(27):22521-22529. doi: 10.1021/acsomega.9b03186. eCollection 2019 Dec 31. ACS Omega. 2019. PMID: 31909335 Free PMC article.
-
Annona squamosa seeds capped calcium oxide nano particles - anti-microbial, antioxidant, anti-ulcer analysis.RSC Adv. 2025 Feb 13;15(7):4904-4914. doi: 10.1039/d5ra00375j. eCollection 2025 Feb 13. RSC Adv. 2025. PMID: 39957829 Free PMC article.
References
-
- Lehninger A.L., Nelson D.L., Cox M.M. In: Lehninger Principles of Biochemistry. 5th ed. Ahr K., editor. W. H. Freeman and Company; New York, NY, USA: 2008.
-
- Hernández-Anzaldo S., Sánchez-Morales N., Alcántara-Flores J.L., Gutiérrez-Pérez R., Zamorano-Ulloa R., Escudero R., de Hoz M.J.R., Reyes-Ortega Y. ESR and magnetic studies of octahedral [Fe(III)(Cl)(pcd)(H2O)(DMSO)] (pcd = pyridine-2,6-dicarboxylato) compound showing Fe(III) species with different spin states in solution. J. Mol. Struct. 2013;1040:22–26. doi: 10.1016/j.molstruc.2013.02.021. - DOI
-
- Reyes-Ortega Y., Alvarez-Toledano C., Ramírez-Rosales D., Sánchez-Sandoval A., González-Vergara E., Zamorano-Ulloa R. Pinch-porphyrins, new spectroscopic and kinetic models of peroxidases. Dalton Trans. 1998:667–674. doi: 10.1039/a704516f. - DOI
-
- Drago R.S. Physical Methods for Chemists. 2nd ed. Saunders College Publishing; Philadelphia, PA, USA: 1992.
-
- Dunford B. Heme Peroxidases. Wiley-VCH; New York, NY, USA: 1999.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

