A Flexible Optical pH Sensor Based on Polysulfone Membranes Coated with pH-Responsive Polyaniline Nanofibers
- PMID: 27355953
- PMCID: PMC4970037
- DOI: 10.3390/s16070986
A Flexible Optical pH Sensor Based on Polysulfone Membranes Coated with pH-Responsive Polyaniline Nanofibers
Abstract
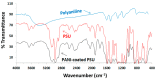
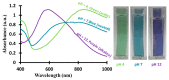
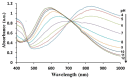
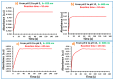
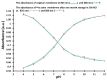
A new optical pH sensor based on polysulfone (PSU) and polyaniline (PANI) was developed. A transparent and flexible PSU membrane was employed as a support. The electrically conductive and pH-responsive PANI was deposited onto the membrane surface by in situ chemical oxidative polymerization (COP). The absorption spectra of the PANI-coated PSU membranes exhibited sensitivity to pH changes in the range of 4-12, which allowed for designing a dual wavelength pH optical sensor. The performance of the membranes was assessed by measuring their response starting from high pH and going down to low pH, and vice versa. It was found that it is necessary to precondition the sensor layers before each measurement due to the slight hysteresis observed during forward and backward pH titrations. PSU membranes with polyaniline coating thicknesses in the range of ≈100-200 nm exhibited fast response times of <4 s, which are attributed to the porous, rough and nanofibrillar morphology of the polyaniline coating. The fabricated pH sensor was characterized by a sigmoidal response (R² = 0.997) which allows for pH determination over a wide dynamic range. All membranes were stable for a period of more than six months when stored in 1 M HCl solution. The reproducibility of the fabricated optical pH sensors was found to be <0.02 absorption units after one month storage in 1 M HCl solution. The performance of the optical pH sensor was tested and the obtained pH values were compared with the results obtained using a pH meter device.
Keywords: chemical oxidative polymerization; flexible pH sensor; nanofibers; optical pH sensor membrane; polyaniline; polysulfone.
Figures



References
-
- Wang X., Kim Y.-G., Drew C., Ku B.-C., Kumar J., Samuelson L.A. Electrostatic assembly of conjugated polymer thin layers on electrospun nanofibrous membranes for biosensors. Nano Lett. 2004;4:331–334. doi: 10.1021/nl034885z. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

