High initial concentration versus low initial concentration sevoflurane for inhalational induction of anaesthesia
- PMID: 27356171
- PMCID: PMC8676071
- DOI: 10.1002/14651858.CD006837.pub3
High initial concentration versus low initial concentration sevoflurane for inhalational induction of anaesthesia
Abstract
Background: Sevoflurane induction for general anaesthesia has been reported to be safe, reliable and well accepted by patients. Sevoflurane induction uses either low or high initial concentrations. The low initial concentration technique involves initially administering a low concentration of sevoflurane and gradually increasing the concentration of the dose until the patient is anaesthetized. The high initial concentration technique involves administering high concentrations from the beginning, then continuing with those high doses until the patient is anaesthetized. This review was originally published in 2013 and has been updated in 2016.
Objectives: We aimed to compare induction times and complication rates between high and low initial concentration sevoflurane anaesthetic induction techniques in adults and children who received inhalational induction for general anaesthesia. We defined 'high' as greater than or equal to and 'low' as less than a 4% initial concentration.
Search methods: For the updated review, we searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 2), MEDLINE (1950 to February 2016), EMBASE (1980 to February 2016), Latin American Caribbean Health Sciences Literature (LILACS) (1982 to February 2016) and the Institute for Scientific Information (ISI) Web of Science (1946 to February 2016). We also searched the reference lists of relevant articles and conference proceedings and contacted the authors of included trials. The original search was run in September 2011.
Selection criteria: We sought all published and unpublished, randomized controlled trials comparing high versus low initial sevoflurane concentration inhalational induction. Our primary outcomes included two measures of anaesthesia (time to loss of the eyelash reflex (LOER) and time until a weighted object held in the patient's hand was dropped), time to successful insertion of a laryngeal mask airway (LMA) and time to endotracheal intubation. Other outcomes were complications of the technique.
Data collection and analysis: We used standardized methods for conducting a systematic review as described in the Cochrane Handbook for Systematic Reviews of Interventions. Two review authors independently extracted details of trial methods and outcome data from reports of all trials considered eligible for inclusion. We conducted all analyses on an intention-to-treat basis, when possible. We estimated overall treatment effects by using a fixed-effect model when we found no substantial heterogeneity, whereas we applied the random-effects model in the presence of considerable heterogeneity.
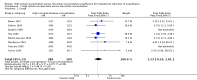
Main results: We reran the searches and included one new study (100 participants) in this updated review. In total, we included 11 studies with 829 participants, although most analyses were based on data from fewer participants and evidence of low quality. We noted substantial heterogeneity in the included trials. Thus, our results should be read with caution. It was not possible to combine trials for the primary outcome (LOER), but individual trials reported faster induction times (typically 24 to 82 seconds faster, 41 seconds (31.37 to 50.62)) with high initial concentration sevoflurane (six studies, 443 participants, low-quality evidence). Apnoea appeared to be more common in the high initial concentration sevoflurane group (risk ratio (RR) 3.14, 95% confidence interval (CI) 1.72 to 5.7, two studies, 160 participants, low-quality evidence). We found no evidence of differences between the two groups in the incidence of cough (odds ratio (OR) 1.23, 95% CI 0.53 to 2.81, eight studies, 589 participants, low-quality evidence), laryngospasm (OR 1.59, 95% CI 0.16 to 15.9, seven studies, 588 participants, low-quality evidence), breath holding (OR 1.16, 95% CI 0.47 to 2.83, five studies, 389 participants, low-quality evidence), patient movement (RR 1.14, 95% CI 0.69 to 1.89, five studies, 445 participants, low-quality evidence) or bradycardia (OR 0.8, 95% CI 0.22 to 2.88, three studies, 199 participants, low-quality evidence), and the overall incidence of complications was low.
Authors' conclusions: A high initial concentration sevoflurane technique probably offers more rapid induction of anaesthesia and a similar rate of complications, except for apnoea, which may be more common with a high initial concentration. However, this conclusion is not definitive because the included studies provided evidence of low quality.
Conflict of interest statement
Polpun Boonmak: none known.
Suhattaya Boonmak: none known.
Porjai Pattanittum: none known.
Figures








Update of
-
High initial concentration versus low initial concentration sevoflurane for inhalational induction of anaesthesia.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD006837. doi: 10.1002/14651858.CD006837.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Jun 29;(6):CD006837. doi: 10.1002/14651858.CD006837.pub3. PMID: 22972100 Updated.
References
References to studies included in this review
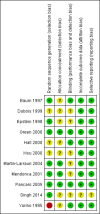
Baum 1997 {published data only}
-
- Baum VC, Yemen TA, Baum LD. Immediate 8% sevoflurane induction in children: a comparison with incremental sevoflurane and incremental halothane. Anesthesia and Analgesia 1997;85(2):313‐6. [PUBMED: 9249106] - PubMed
Dubois 1999 {published data only}
-
- Dubois MC, Piat V, Constant I, Lamblin O, Murat I. Comparison of three techniques for induction of anaesthesia with sevoflurane in children. Paediatric Anaesthesia 1999;9(1):19‐23. [PUBMED: 10712710] - PubMed
Epstein 1998 {published data only}
-
- Epstein RH, Stein AL, Marr AT, Lessin JB. High concentration versus incremental induction of anesthesia with sevoflurane in children: a comparison of induction times, vital signs, and complications. Journal of Clinical Anesthesia 1998;10(1):41‐5. [PUBMED: 9526937] - PubMed
Green 2000 {published data only}
-
- Green DH, Townsend P, Bagshaw O, Stokes MA. Nodal rhythm and bradycardia during inhalation induction with sevoflurane in infants: a comparison of incremental and high‐concentration techniques. British Journal of Anaesthesia 2000;85(3):368‐70. [PUBMED: 11103176] - PubMed
Hall 2000 {published data only}
-
- Hall JE, Ebert TJ, Harmer M. Induction characteristics with 3% and 8% sevoflurane in adults: an evaluation of the second stage of anaesthesia and its haemodynamic consequences. Anaesthesia 2000;55(6):545‐50. [PUBMED: 10866717] - PubMed
Hsu 2000 {published data only}
-
- Hsu YW, Pan MH, Huang CJ, Cheng CR, Wu KH, Wei TT. Comparison of inhalation induction with 2%, 4%, 6%, and 8% sevoflurane in nitrous oxide for pediatric patients. Acta Anaesthesiologica Sinica 2000;38(2):73‐8. [PUBMED: 11000669] - PubMed
Martin‐Larrauri 2004 {published data only}
-
- Martin‐Larrauri R, Gilsanz F, Rodrigo J, Vila P, Ledesma M, Casimiro C. Conventional stepwise vs. vital capacity rapid inhalation induction at two concentrations of sevoflurane. European Journal of Anaesthesiology 2004;21(4):265‐71. [PUBMED: 15109188] - PubMed
Mendonca 2001 {published data only}
-
- Mendonca C, Thorpe C. Effect of smoking on induction of anaesthesia with sevoflurane. Anaesthesia 2001;56(1):19‐23. [PUBMED: 11167431] - PubMed
Pancaro 2005 {published data only}
-
- Pancaro C, Giovannoni S, Toscano A, Peduto VA. Apnea during induction of anesthesia with sevoflurane is related to its mode of administration. Canadian Journal of Anaesthesia 2005;52(6):591‐4. [PUBMED: 15983143] - PubMed
Singh 2014 {published data only}
-
- Singh PM, Trikha A, Sinha R, Rewari V, Ramachandran R, Borle A. Sevoflurane induction procedure: cost comparison between fixed 8% versus incremental techniques in pediatric patients. AANA Journal 2014;82(1):32‐7. [PUBMED: 24654350] - PubMed
Yurino 1995 {published data only}
-
- Yurino M, Kimura H. Efficient inspired concentration of sevoflurane for vital capacity rapid inhalation induction (VCRII) technique. Journal of Clinical Anesthesia 1995;7(3):228‐31. [PUBMED: 7669314] - PubMed
References to studies excluded from this review
Julliac 2013 {published data only}
-
- Julliac B, Cotillon P, Guehl D, Richez B, Sztark F. Target‐controlled induction with 2.5% sevoflurane does not avoid the risk of electroencephalographic abnormalities. Annales Françaises d'Anesthésie et de Réanimation 2013;32(10):e143‐8. [PUBMED: 24035611] - PubMed
Kreuzer 2014 {published data only}
Lee 2013 {published data only}
-
- Lee SY, Cheng SL, Ng SB, Lim SL. Single‐breath vital capacity high concentration sevoflurane induction in children: with or without nitrous oxide?. British Journal of Anaesthesia 2013;110(1):81‐6. [PUBMED: 22986418] - PubMed
Munoz 1999 {published data only}
-
- Munoz HR, Gonzalez JA, Concha MR, Palma MA. Hemodynamic response to tracheal intubation after vital capacity rapid inhalation induction (VCRII) with different concentrations of sevoflurane. Journal of Clinical Anesthesia 1999;11(7):567‐71. [PUBMED: 10624641] - PubMed
Nishiyama 1997 {published data only}
-
- Nishiyama T, Aibiki M, Hanaoka K. Haemodynamic and catecholamine changes during rapid sevoflurane induction with tidal volume breathing. Canadian Journal of Anaesthesia 1997;44(10):1066‐70. [PUBMED: 9350365] - PubMed
Walpole 1999 {published data only}
-
- Walpole R, Logan M. Effect of sevoflurane concentration on inhalation induction of anaesthesia in the elderly. British Journal of Anaesthesia 1999;82(1):20‐4. [PUBMED: 10325830] - PubMed
Additional references
Aantaa 2001
-
- Aantaa R, Takala R, Muittari P. Sevoflurane EC50 and EC95 values for laryngeal mask insertion and tracheal intubation in children. British Journal of Anaesthesia 2001;86(2):213‐6. [PUBMED: 11573662] - PubMed
Bai 2010
-
- Bai W, Voepel‐Lewis T, Malviya S. Hemodynamic changes in children with Down syndrome during and following inhalation induction of anesthesia with sevoflurane. Journal of Clinical Anesthesia 2010;22(8):592‐7. [PUBMED: 21109130] - PubMed
Baker 1999
-
- Baker CE, Smith I. Sevoflurane: a comparison between vital capacity and tidal breathing techniques for the induction of anaesthesia and laryngeal mask airway placement. Anaesthesia 1999;54(9):841‐4. [PUBMED: 10460554] - PubMed
Banchereau 2005
-
- Banchereau F, Herve Y, Quinart A, Cros AM. Pressure support ventilation during inhalational induction with sevoflurane and remifentanil in adults. European Journal of Anaesthesiology 2005;22(11):826‐30. [PUBMED: 16225715] - PubMed
Bordes 2006
-
- Bordes M, Cros AM. Inhalation induction with sevoflurane in paediatrics: what is new?. Annales Françaises d'Anesthèsie et de Rèanimation 2006;25(4):413‐6. [PUBMED: 16455225] - PubMed
Constant 2005
-
- Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Paediatric Anaesthesia 2005;15(4):266‐74. [PUBMED: 15787916] - PubMed
De Hert 2015
Delgado‐Herrera 2001
Eger 2003
-
- Eger EI 2nd, Eisenkraft JB, Weiskopf RB. The Pharmacology of Inhaled Anesthetics. 3rd Edition. New Jersey: Baxter Healthcare Corporation, 2003.
Fassoulaki 2015
Fernandez‐Alcantud 2008
-
- Fernandez‐Alcantud J, Sanabria Carretero P, Rodriguez Perez E, Planas Roca A. Anesthetic induction with nitrous‐oxide‐free sevoflurane in pediatric patients [Induccion anestesica con sevoflurano libre de oxido nitroso en pediatria]. Revista Espanola de Anestesiologia y Reanimacion 2008;55(2):69‐74. [PUBMED: 18383967] - PubMed
Ghatge 2003
-
- Ghatge S, Lee J, Smith I. Sevoflurane: an ideal agent for adult day‐case anesthesia?. Acta Anaesthesiologica Scandinavica 2003;47(8):917‐31. [PUBMED: 12904182] - PubMed
Gibert 2012
-
- Gibert S, Sabourdin N, Louvet N, Moutard ML, Piat V, Guye ML, et al. Epileptogenic effect of sevoflurane: determination of the minimal alveolar concentration of sevoflurane associated with major epileptoid signs in children. Anesthesiology 2012;117(6):1253‐61. [PUBMED: 23103557] - PubMed
Goldman 2003
-
- Goldman LJ. Anesthetic uptake of sevoflurane and nitrous oxide during an inhaled induction in children. Anesthesia and Analgesia 2003;96(2):400‐6. [PUBMED: 12538185] - PubMed
Goresky 1996
-
- Goresky GV, Muir J. Inhalation induction of anaesthesia. Canadian Journal of Anaesthesia 1996;43(11):1085‐9. [PUBMED: 8922761] - PubMed
GRADEpro 2011 [Computer program]
-
- Brozek J, Oxman A, Schunemann H. GRADEpro. http://www.cc‐ims.net/revman/gradepro. Version 3.6.1 for Windows. Brozek J, Oxman A, Schunemann H, 2011.
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kaisti 1999
-
- Kaisti KK, Jaaskelainen SK, Rinne JO, Metsahonkala L, Scheinin H. Epileptiform discharges during 2 MAC sevoflurane anesthesia in two healthy volunteers. Anesthesiology 1999;91(6):1952‐5. [EMBASE: 10598642] - PubMed
Kihara 2003
-
- Kihara S, Yaguchi Y, Inomata S, Watanabe S, Brimacombe JR, Taguchi N, et al. Influence of nitrous oxide on minimum alveolar concentration of sevoflurane for laryngeal mask insertion in children. Anesthesiology 2003;99(5):1055‐8. [PUBMED: 14576538] - PubMed
Kraemer 2010
-
- Kraemer FW, Stricker PA, Gurnaney HG, McClung H, Meador MR, Sussman E, et al. Bradycardia during induction of anesthesia with sevoflurane in children with Down syndrome. Anesthesia and Analgesia 2010;111(5):1259‐63. [PUBMED: 20736433] - PubMed
Lejus 2006
-
- Lejus C, Bazin V, Fernandez M, Nguyen JM, Radosevic A, Quere MF, et al. Inhalation induction using sevoflurane in children: the single‐breath vital capacity technique compared to the tidal volume technique. Anaesthesia 2006; Vol. 61, issue 6:535‐40. - PubMed
Lerman 2009
-
- Lerman J, Johr M. Inhalational anesthesia vs total intravenous anesthesia (TIVA) for pediatric anesthesia. Paediatric Anaesthesia 2009;19(5):521‐34. [PUBMED: 19453585] - PubMed
Liu 2010
-
- Liu SJ, Li Y, Sun B, Wang CS, Gong YL, Zhou YM, et al. A comparison between vital capacity induction and tidal breathing induction techniques for the induction of anesthesia and compound A production. Chinese Medical Journal 2010;123(17):2336‐40. [PUBMED: 21034545] - PubMed
Meaudre 2004
-
- Meaudre E, Boret H, Suppini A, Sallaberry M, Benefice S, Palmier B. Sufentanil supplementation of sevoflurane during induction of anaesthesia: a randomized study. European Journal of Anaesthesiology 2004;21(10):793‐6. [PUBMED: 15678734] - PubMed
Merquiol 2006
-
- Merquiol F, Humblot A, Fares M, Moutard ML, Murat I, Constant I. EEG epileptoid signs during sevoflurane induction in children: a comparative study between incremental and rapid induction. European Journal of Anaesthesiology 2006; Vol. 23, issue suppl 37:1.
Mizrak 2013
-
- Mizrak A, Ganidagli S, Cengiz MT, Oner U, Saricicek V. The effects of DEX premedication on volatile induction of mask anesthesia (VIMA) and sevoflurane requirements. Journal of Clinical Monitoring and Computing 2013;27(3):239‐34. [PUBMED: 23400425] - PubMed
Nathan 2004
-
- Nathan N, Bazin JE, Cros AM. [Inhalation induction] [Induction par inhalation]. Annales Francaises d'Anesthesie et de Reanimation 2004;23(9):884‐99. [PUBMED: 15471636] - PubMed
Nishiyama 2002
-
- Nishiyama T, Matsukawa T, Yokoyama T, Hanaoka K. Rapid inhalation induction with 7% sevoflurane combined with intravenous midazolam. Journal of Clinical Anesthesia 2002;14(4):290‐5. [PUBMED: 12088814] - PubMed
O'Shea 2001
-
- O'Shea H, Moultrie S, Drummond GB. Influence of nitrous oxide on induction of anaesthesia with sevoflurane. British Journal of Anaesthesia 2001;87(2):286‐8. [PUBMED: 11493504] - PubMed
RevMan 5.3 [Computer program]
-
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roodman 2003
-
- Roodman S, Bothwell M, Tobias JD. Bradycardia with sevoflurane induction in patients with trisomy 21. Paediatric Anaesthesia 2003;13(6):538‐40. [PUBMED: 12846713] - PubMed
Schultz 2012
Siau 2002
-
- Siau C, Liu EH. Nitrous oxide does not improve sevoflurane induction of anesthesia in adults. Journal of Clinical Anesthesia 2002;14(3):218‐22. [PUBMED: 12031757] - PubMed
Smith 2016
-
- Smith DA, Bath J. Epileptiform activity during induction of anesthesia with sevoflurane prior to elective carotid endarterectomy. Vascular 2016;24(1):96‐9. [PUBMED: 25687719] - PubMed
Vakkuri 2001
-
- Vakkuri A, Yli‐Hankala A, Sarkela M, Lindgren L, Mennander S, Korttila K, et al. Sevoflurane mask induction of anaesthesia is associated with epileptiform EEG in children. Acta Anaesthesiologica Scandinavica 2001;45(7):805‐11. [PUBMED: 11472278] - PubMed
van den Berg 2005
-
- Berg AA, Chitty DA, Jones RD, Sohel MS, Shahen A. Intravenous or inhaled induction of anesthesia in adults? An audit of preoperative patient preferences. Anesthesia and Analgesia 2005;100(5):1422‐4. [PUBMED: 15845699] - PubMed
Walia 2016
-
- Walia H, Ruda J, Tobias JD. Sevoflurane and bradycardia in infants with trisomy 21: a case report and review of the literature. International Journal of Pediatric Otorhinolaryngology 2016;80(1):5‐7. [PUBMED: 26746603] - PubMed
Watanabe 2006
-
- Watanabe T, Inagaki Y, Ishibe Y. Clonidine premedication effects on inhaled induction with sevoflurane in adults: a prospective, double‐blind, randomized study. Acta Anaesthesiologica Scandinavica 2006;50(2):180‐7. [PUBMED: 16430539] - PubMed
Yao 2015
-
- Yao Y, Qian B, Lin Y, Wu W, Ye H, Chen Y. Intranasal dexmedetomidine premedication reduces minimum alveolar concentration of sevoflurane for laryngeal mask airway insertion and emergence delirium in children: a prospective, randomized, double‐blind, placebo‐controlled trial. Paediatric Anaesthesia 2015;25(5):492‐8. [PUBMED: 25487567] - PubMed
References to other published versions of this review
Boonmak 2007
-
- Boonmak P, Boonmak S, Krisanaprakornkit W, Pattanittum P. High concentration versus low concentration sevoflurane for anaesthesia induction. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006837] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

