Aurora kinase A revives dormant laryngeal squamous cell carcinoma cells via FAK/PI3K/Akt pathway activation
- PMID: 27356739
- PMCID: PMC5217022
- DOI: 10.18632/oncotarget.10233
Aurora kinase A revives dormant laryngeal squamous cell carcinoma cells via FAK/PI3K/Akt pathway activation
Abstract
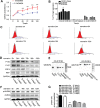
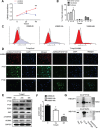
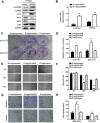
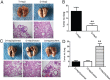
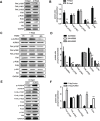
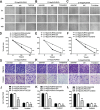
Revival of dormant tumor cells may be an important tumor metastasis mechanism. We hypothesized that aurora kinase A (AURKA), a cell cycle control kinase, promotes the transition of laryngeal squamous cell carcinoma (LSCC) cells from G0 phase to active division. We therefore investigated whether AURKA could revive dormant tumor cells to promote metastasis. Western blotting revealed that AURKA expression was persistently low in dormant laryngeal cancer Hep2 (D-Hep2) cells and high in non-dormant (T-Hep2) cells. Decreasing AURKA expression in T-Hep2 cells induced dormancy and reduced FAK/PI3K/Akt pathway activity. Increasing AURKA expression in D-Hep2 cells increased FAK/PI3K/Akt pathway activity and enhanced cellular proliferation, migration, invasion and metastasis. In addition, FAK/PI3K/Akt pathway inhibition caused dormancy-like behavior and reduced cellular mobility, migration and invasion. We conclude that AURKA may revive dormant tumor cells via FAK/PI3K/Akt pathway activation, thereby promoting migration and invasion in laryngeal cancer. AURKA/FAK/PI3K/Akt inhibitors may thus represent potential targets for clinical LSCC treatment.
Keywords: Akt; FAK; PI3K; aurora kinase A; laryngeal cancer.
Conflict of interest statement
All authors declare no conflicts interest.
Figures

Similar articles
-
Activation of the FAK/PI3K pathway is crucial for AURKA-induced epithelial-mesenchymal transition in laryngeal cancer.Oncol Rep. 2016 Aug;36(2):819-26. doi: 10.3892/or.2016.4872. Epub 2016 Jun 13. Oncol Rep. 2016. PMID: 27373675
-
Aurora kinase A induces chemotherapy resistance through revival of dormant cells in laryngeal squamous cell carcinoma.Head Neck. 2019 Jul;41(7):2239-2248. doi: 10.1002/hed.25689. Epub 2019 Feb 1. Head Neck. 2019. PMID: 30706572
-
AURKA promotes cell migration and invasion of head and neck squamous cell carcinoma through regulation of the AURKA/Akt/FAK signaling pathway.Oncol Lett. 2016 Mar;11(3):1889-1894. doi: 10.3892/ol.2016.4110. Epub 2016 Jan 14. Oncol Lett. 2016. PMID: 26998095 Free PMC article.
-
The PI3K/Akt pathway as a target in the treatment of hematologic malignancies.Anticancer Agents Med Chem. 2009 Jun;9(5):550-9. doi: 10.2174/187152009788451851. Anticancer Agents Med Chem. 2009. PMID: 19519296 Review.
-
Synthesis and biological activity of Akt/PI3K inhibitors.Mini Rev Med Chem. 2006 Oct;6(10):1127-36. doi: 10.2174/138955706778560139. Mini Rev Med Chem. 2006. PMID: 17073713 Review.
Cited by
-
Network analysis revealed aurora kinase dysregulation in five gynecological types of cancer.Oncol Lett. 2018 Jan;15(1):1125-1132. doi: 10.3892/ol.2017.7368. Epub 2017 Nov 8. Oncol Lett. 2018. PMID: 29391900 Free PMC article.
-
Revealing the active ingredients and mechanisms of Xiatianwu against hepatocellular carcinoma: a study based on network pharmacology and bioinformatics.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jan;398(1):729-746. doi: 10.1007/s00210-024-03278-2. Epub 2024 Jul 25. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39052060
-
IGF1R and Src inhibition induce synergistic cytotoxicity in HNSCC through inhibition of FAK.Sci Rep. 2021 May 24;11(1):10826. doi: 10.1038/s41598-021-90289-1. Sci Rep. 2021. PMID: 34031486 Free PMC article.
-
Combined inhibition of AURKA and HSF1 suppresses proliferation and promotes apoptosis in hepatocellular carcinoma by activating endoplasmic reticulum stress.Cell Oncol (Dordr). 2021 Oct;44(5):1035-1049. doi: 10.1007/s13402-021-00617-w. Epub 2021 Jun 26. Cell Oncol (Dordr). 2021. PMID: 34176092
-
Aurora kinase A promotes epithelial‑mesenchymal transition by regulating P130 and P107 molecules in thyroid cancer cells.Exp Ther Med. 2025 Mar 12;29(5):93. doi: 10.3892/etm.2025.12843. eCollection 2025 May. Exp Ther Med. 2025. PMID: 40162054 Free PMC article.
References
-
- Bingol F, Yoruk O, Bingol BO, Erdemci B, Ozkan O, Mazlumoglu MR. Estimation of the efficacy of chemo-radiotherapy on tumor regression in the patients with laryngeal cancer via computerized tomography using the Cavalieri method. Acta Otolaryngol. 2015;21:1–4. - PubMed
-
- Yu GP, Mehta V, Branovan D. Improved survival among patients with base of tongue and tonsil cancer in the United States. Cancer Causes Control. 2012;23:153–64. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

