IL-17 down-regulates the immunosuppressive capacity of olfactory ecto-mesenchymal stem cells in murine collagen-induced arthritis
- PMID: 27356747
- PMCID: PMC5189999
- DOI: 10.18632/oncotarget.10261
IL-17 down-regulates the immunosuppressive capacity of olfactory ecto-mesenchymal stem cells in murine collagen-induced arthritis
Abstract
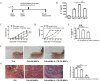
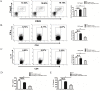
Olfactory ecto-mesenchymal stem cells (OE-MSCs) are a population of cells which has been recognized as a new resident stem cell type in the olfactory lamina propria. OE-MSCs have been shown to exert their immunosuppressive capacity by modulating T cell responses, including up-regulation of regulatory T cells (Tregs) and down-regulation of Th1/Th17 cells. As an inflammatory cytokine, IL-17 plays a critical role in orchestrating the inflammatory response during the development of collagen-induced arthritis (CIA). However, it is unclear whether the increased level of IL-17 may affect the immunosuppressive function of OE-MSCs under inflammatory condition. In this study, we found that IL-17 could significantly reduce the suppressive capacity of OE-MSCs on CD4+ T cells and down-regulate the suppressive factors produced by OE-MSCs. Notably, IL-17 treatment abolished the capacity of OE-MSCs in inducing Treg expansion. In addition, knockdown of IL-17R in OE-MSCs significantly enhanced their therapeutic effect in ameliorating CIA upon adoptive transfer. Moreover, IL-17R knockdown-OE-MSCs could efficiently induce Tregs expansion and reduce Th1 and Th17 responses. Taken together, all these data suggest that IL-17R knockdown in OE-MSCs may provide a novel strategy in maintaining their immunosuppressive properties for the treatment of autoimmune diseases.
Keywords: IL-17; Immune response; Immunity; Immunology and Microbiology Section; Treg; collagen-induced arthritis; olfactory ecto-mesenchymal stem cells; suppressive capacity.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



Similar articles
-
Olfactory ecto-mesenchymal stem cells possess immunoregulatory function and suppress autoimmune arthritis.Cell Mol Immunol. 2016 May;13(3):401-8. doi: 10.1038/cmi.2015.82. Epub 2015 Sep 21. Cell Mol Immunol. 2016. PMID: 26388237 Free PMC article.
-
Olfactory Ecto-Mesenchymal Stem Cell-Derived Exosomes Ameliorate Experimental Colitis via Modulating Th1/Th17 and Treg Cell Responses.Front Immunol. 2020 Dec 10;11:598322. doi: 10.3389/fimmu.2020.598322. eCollection 2020. Front Immunol. 2020. PMID: 33362781 Free PMC article.
-
Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells.Arthritis Rheum. 2009 Apr;60(4):1006-19. doi: 10.1002/art.24405. Arthritis Rheum. 2009. PMID: 19333946
-
Regulation of immune responses by interleukin-27.Immunol Rev. 2008 Dec;226:234-47. doi: 10.1111/j.1600-065X.2008.00710.x. Immunol Rev. 2008. PMID: 19161428 Review.
-
Mesenchymal stem cells overexpressing IL-35: a novel immunosuppressive strategy and therapeutic target for inducing transplant tolerance.Stem Cell Res Ther. 2018 Sep 26;9(1):254. doi: 10.1186/s13287-018-0988-9. Stem Cell Res Ther. 2018. PMID: 30257721 Free PMC article. Review.
Cited by
-
Mesenchymal Stem/Stromal Cells for Rheumatoid Arthritis Treatment: An Update on Clinical Applications.Cells. 2020 Aug 7;9(8):1852. doi: 10.3390/cells9081852. Cells. 2020. PMID: 32784608 Free PMC article. Review.
-
IL-17 Triggers Invasive and Migratory Properties in Human MSCs, while IFNy Favors their Immunosuppressive Capabilities: Implications for the "Licensing" Process.Stem Cell Rev Rep. 2020 Dec;16(6):1266-1279. doi: 10.1007/s12015-020-10051-4. Epub 2020 Oct 16. Stem Cell Rev Rep. 2020. PMID: 33067729 Free PMC article.
-
Mesenchymal Stromal Cells from the Epidermis and Dermis of Psoriasis Patients: Morphology, Immunophenotype, Differentiation Patterns, and Regulation of T Cell Proliferation.Stem Cells Int. 2019 Dec 1;2019:4541797. doi: 10.1155/2019/4541797. eCollection 2019. Stem Cells Int. 2019. PMID: 31885608 Free PMC article.
-
IL17/IL17RA as a Novel Signaling Axis Driving Mesenchymal Stem Cell Therapeutic Function in Experimental Autoimmune Encephalomyelitis.Front Immunol. 2018 Apr 30;9:802. doi: 10.3389/fimmu.2018.00802. eCollection 2018. Front Immunol. 2018. PMID: 29760692 Free PMC article.
-
Inflammatory niche: Mesenchymal stromal cell priming by soluble mediators.World J Stem Cells. 2020 Sep 26;12(9):922-937. doi: 10.4252/wjsc.v12.i9.922. World J Stem Cells. 2020. PMID: 33033555 Free PMC article. Review.
References
-
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. - PubMed
-
- Kageyama Y, Koide Y, Yoshida A, Uchijima M, Arai T, Miyamoto S, Ozeki T, Hiyoshi M, Kushida K, Inoue T. Reduced susceptibility to collagen-induced arthritis in mice deficient in IFN-gamma receptor. J Immunol. 1998;161:1542–1548. - PubMed
-
- Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. - PubMed
-
- Mauri C, Feldmann M, Williams RO. Down-regulation of Th1-mediated pathology in experimental arthritis by stimulation of the Th2 arm of the immune response. Arthritis Rheum. 2003;48:839–845. - PubMed
-
- Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR, Toes RE. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–2221. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

