Establishment and Reversal of HIV-1 Latency in Naive and Central Memory CD4+ T Cells In Vitro
- PMID: 27356901
- PMCID: PMC5008097
- DOI: 10.1128/JVI.00553-16
Establishment and Reversal of HIV-1 Latency in Naive and Central Memory CD4+ T Cells In Vitro
Abstract
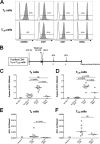
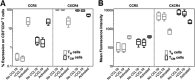
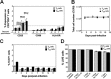
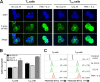
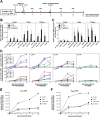
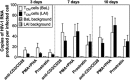
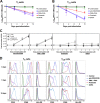
The latent HIV-1 reservoir primarily resides in resting CD4(+) T cells which are a heterogeneous population composed of both naive (TN) and memory cells. In HIV-1-infected individuals, viral DNA has been detected in both naive and memory CD4(+) T cell subsets although the frequency of HIV-1 DNA is typically higher in memory cells, particularly in the central memory (TCM) cell subset. TN and TCM cells are distinct cell populations distinguished by many phenotypic and physiological differences. In this study, we used a primary cell model of HIV-1 latency that utilizes direct infection of highly purified TN and TCM cells to address differences in the establishment and reversal of HIV-1 latency. Consistent with what is seen in vivo, we found that HIV-1 infected TN cells less efficiently than TCM cells. However, when the infected TN cells were treated with latency-reversing agents, including anti-CD3/CD28 antibodies, phorbol myristate acetate/phytohemagglutinin, and prostratin, as much (if not more) extracellular virion-associated HIV-1 RNA was produced per infected TN cell as per infected TCM cell. There were no major differences in the genomic distribution of HIV-1 integration sites between TN and TCM cells that accounted for these observed differences. We observed decay of the latent HIV-1 cells in both T cell subsets after exposure to each of the latency-reversing agents. Collectively, these data highlight significant differences in the establishment and reversal of HIV-1 latency in TN and TCM CD4(+) T cells and suggest that each subset should be independently studied in preclinical and clinical studies.
Importance: The latent HIV-1 reservoir is frequently described as residing within resting memory CD4(+) T cells. This is largely due to the consistent finding that memory CD4(+) T cells, specifically the central (TCM) and transitional memory compartments, harbor the highest levels of HIV-1 DNA in individuals on suppressive therapy. This has yielded little research into the contribution of CD4(+) naive T (TN) cells to the latent reservoir. In this study, we show that although TN cells harbor significantly lower levels of HIV-1 DNA, following latency reversal, they produced as many virions as did the TCM cells (if not more virions). This suggests that latently infected TN cells may be a major source of virus following treatment interruption or failure. These findings highlight the need for a better understanding of the establishment and reversal of HIV-1 latency in TN cells in evaluating therapeutic approaches to eliminate the latent reservoir.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Schacker T, Little S, Connick E, Gebhard K, Zhang ZQ, Krieger J, Pryor J, Havlir D, Wong JK, Schooley RT, Richman D, Corey L, Haase AT. 2001. Productive infection of T cells in lymphoid tissues during primary and early human immunodeficiency virus infection. J Infect Dis 183:555–562. doi: 10.1086/318524. - DOI - PubMed
-
- Schacker T, Little S, Connick E, Gebhard-Mitchell K, Zhang ZQ, Krieger J, Pryor J, Havlir D, Wong JK, Richman D, Corey L, Haase AT. 2000. Rapid accumulation of human immunodeficiency virus (HIV) in lymphatic tissue reservoirs during acute and early HIV infection: implications for timing of antiretroviral therapy. J Infect Dis 181:354–357. doi: 10.1086/315178. - DOI - PubMed
-
- Archin NM, Vaidya NK, Kuruc JD, Liberty AL, Wiegand A, Kearney MF, Cohen MS, Coffin JM, Bosch RJ, Gay CL, Eron JJ, Margolis DM, Perelson AS. 2012. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci U S A 109:9523–9528. doi: 10.1073/pnas.1120248109. - DOI - PMC - PubMed
-
- Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, Smith JY, Brinkman AL, Peter LE, Mathew SI, Smith KM, Borducchi EN, Rosenbloom DI, Lewis MG, Hattersley J, Li B, Hesselgesser J, Geleziunas R, Robb ML, Kim JH, Michael NL, Barouch DH. 2014. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512:74–77. doi: 10.1038/nature13594. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

