Parasympathetic innervation of vertebrobasilar arteries: is this a potential clinical target?
- PMID: 27357059
- PMCID: PMC5108906
- DOI: 10.1113/JP272450
Parasympathetic innervation of vertebrobasilar arteries: is this a potential clinical target?
Abstract
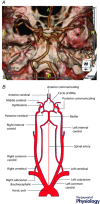
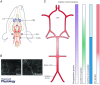
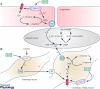
This review aims to summarise the contemporary evidence for the presence and function of the parasympathetic innervation of the cerebral circulation with emphasis on the vertebral and basilar arteries (the posterior cerebral circulation). We consider whether the parasympathetic innervation of blood vessels could be used as a means to increase cerebral blood flow. This may have clinical implications for pathologies associated with cerebral hypoperfusion such as stroke, dementia and hypertension. Relative to the anterior cerebral circulation little is known of the origins and neurochemical phenotypes of the parasympathetic innervation of the vertebrobasilar arteries. These vessels normally provide blood flow to the brainstem and cerebellum but can, via the Circle of Willis upon stenosis of the internal carotid arteries, supply blood to the anterior cerebral circulation too. We review the multiple types of parasympathetic fibres and their distinct transmitter mechanisms and how these vary with age, disease and species. We highlight the importance of parasympathetic fibres for mediating the vasodilatory response to sympathetic activation. Current trials are investigating the possibility of electrically stimulating the postganglionic parasympathetic ganglia to improve cerebal blood flow to reduce the penumbra following stroke. We conclude that although there are substantial gaps in our understanding of the origins of parasympathetic innervation of the vertebrobasilar arteries, activation of this system under some conditions might bring therapeutic benefits.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures


References
-
- Akiho H, Chijiiwa Y, Okabe H, Harada N & Nawata H (1995). Interaction between atrial natriuretic peptide and vasoactive intestinal peptide in guinea pig cecal smooth muscle. Gastroenterology 109, 1105–1112. - PubMed
-
- Alpers BJ, Berry RG & Paddison RM (1959). Anatomical studies of the circle of Willis in normal brain. AMA Arch Neurol Psychiatry 81, 409–418. - PubMed
-
- Andersson B & Jewell P (1956). The distribution of carotid and vertebral blood in the brain and spinal cord of the goat. Q J Exp Physiol Cogn Med Sci 41, 462–474.
-
- Ando K (1988). Distribution and origin of vasoactive intestinal polypeptide (VIP)‐immunoreactive, acetylcholinesterase‐positive and adrenergic nerves of the cerebral arteries in the bent‐winged bat (Mammalia: Chiroptera). Cell Tissue Res 251, 345–351. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

