Unfavorable neuroblastoma prognostic factor NLRR2 inhibits cell differentiation by transcriptional induction through JNK pathway
- PMID: 27357360
- PMCID: PMC5021041
- DOI: 10.1111/cas.13003
Unfavorable neuroblastoma prognostic factor NLRR2 inhibits cell differentiation by transcriptional induction through JNK pathway
Abstract
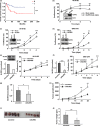
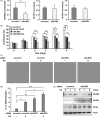
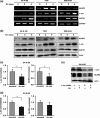
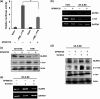
The novel human gene family encoding neuronal leucine rich repeat (NLRR) proteins were identified as prognostic markers from our previous screening of primary neuroblastoma (NB) cDNA libraries. Of the NLRR gene family members, NLRR1 and NLRR3 are associated with the regulation of cellular proliferation and differentiation, respectively. However, the functional regulation and clinical significance of NLRR2 in NB remain unclear. Here, we evaluated the differential expression of NLRR2, where high expressions of NLRR2 were significantly associated with a poor prognosis of NB (P = 0.0009), in 78 NBs. Enforced expression of NLRR2 in NB cells enhanced cellular proliferation and induced resistance to retinoic acid (RA)-mediated cell growth inhibition. In contrast, knockdown of NLRR2 exhibited growth inhibition effects and enhanced RA-induced cell differentiation in NB cells. After RA treatment, NLRR2 expression was increased and correlated with the upregulation of c-Jun, a member of the activator protein-1 (AP-1) family in NB cells. Moreover, the expressions of NLRR2 and c-Jun were suppressed by treatment with a JNK inhibitor, which ameliorated the promoter activity of the NLRR2 gene while knockdown of c-Jun reduced NLRR2 expression. We then searched AP-1 binding consensus in the NLRR2 promoter region and confirmed c-Jun recruitment at a consensus. Conclusively, NLRR2 must be an inducible gene regulated by the JNK pathway to enhance cell survival and inhibit NB cell differentiation. Therefore, NLRR2 should have an important role in NB aggressiveness and be a potential therapeutic target for the treatment of RA resistant and aggressive NB.
Keywords: JNK; NLRR2; c-Jun; differentiation; neuroblastoma.
© 2016 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures



Similar articles
-
Expression of NLRR3 orphan receptor gene is negatively regulated by MYCN and Miz-1, and its downregulation is associated with unfavorable outcome in neuroblastoma.Clin Cancer Res. 2011 Nov 1;17(21):6681-92. doi: 10.1158/1078-0432.CCR-11-0313. Epub 2011 Sep 9. Clin Cancer Res. 2011. PMID: 21908575
-
Identification of novel human neuronal leucine-rich repeat (hNLRR) family genes and inverse association of expression of Nbla10449/hNLRR-1 and Nbla10677/hNLRR-3 with the prognosis of primary neuroblastomas.Int J Oncol. 2004 Jun;24(6):1457-66. Int J Oncol. 2004. PMID: 15138588
-
Induction of cyclin-dependent kinase 5 and its activator p35 through the extracellular-signal-regulated kinase and protein kinase A pathways during retinoic-acid mediated neuronal differentiation in human neuroblastoma SK-N-BE(2)C cells.J Neurochem. 2004 Nov;91(3):634-47. doi: 10.1111/j.1471-4159.2004.02770.x. J Neurochem. 2004. PMID: 15485494
-
HMGA molecules in neuroblastic tumors.Ann N Y Acad Sci. 2004 Dec;1028:122-32. doi: 10.1196/annals.1322.013. Ann N Y Acad Sci. 2004. PMID: 15650238 Review.
-
WNT signaling, the development of the sympathoadrenal-paraganglionic system and neuroblastoma.Cell Mol Life Sci. 2018 Mar;75(6):1057-1070. doi: 10.1007/s00018-017-2685-8. Epub 2017 Oct 22. Cell Mol Life Sci. 2018. PMID: 29058015 Free PMC article. Review.
Cited by
-
Role of Key Micronutrients from Nutrigenetic and Nutrigenomic Perspectives in Cancer Prevention.Medicina (Kaunas). 2019 Jun 18;55(6):283. doi: 10.3390/medicina55060283. Medicina (Kaunas). 2019. PMID: 31216637 Free PMC article. Review.
-
High-Throughput Screening of Mouse Gene Knockouts Identifies Established and Novel High Body Fat Phenotypes.Diabetes Metab Syndr Obes. 2021 Aug 28;14:3753-3785. doi: 10.2147/DMSO.S322083. eCollection 2021. Diabetes Metab Syndr Obes. 2021. PMID: 34483672 Free PMC article.
-
Regorafenib is effective against neuroblastoma in vitro and in vivo and inhibits the RAS/MAPK, PI3K/Akt/mTOR and Fos/Jun pathways.Br J Cancer. 2020 Aug;123(4):568-579. doi: 10.1038/s41416-020-0905-8. Epub 2020 May 27. Br J Cancer. 2020. PMID: 32457362 Free PMC article.
-
Activation of PERK Contributes to Apoptosis and G2/M Arrest by Microtubule Disruptors in Human Colorectal Carcinoma Cells ‡.Cancers (Basel). 2019 Dec 30;12(1):97. doi: 10.3390/cancers12010097. Cancers (Basel). 2019. PMID: 31906029 Free PMC article.
-
Prognostic value and biological function of LRRN4 in colorectal cancer.Cancer Cell Int. 2022 Apr 19;22(1):158. doi: 10.1186/s12935-022-02579-x. Cancer Cell Int. 2022. PMID: 35440048 Free PMC article.
References
-
- Chang HH, Lee H, Hu MK et al Notch1 expression predicts an unfavorable prognosis and serves as a therapeutic target of patients with neuroblastoma. Clin Cancer Res 2010; 16: 4411–20. - PubMed
-
- Brodeur GM, Nakagawara A. Molecular basis of clinical heterogeneity in Neuroblastoma. Am J Pediatr Hematol Oncol 1992; 14: 111–6. - PubMed
-
- Nakagawara A, Arima M, Azar CG, Scavarda NJ, Brodeur GM. Inverse relationship between trk expression and N‐myc amplification in human neuroblastomas. Cancer Res 1992; 52: 1364–8. - PubMed
-
- Chen Y, Takita J, Choi YL et al Oncogenic mutations of ALK kinase in neuroblastoma. Nature 2008; 16: 971–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

