Trial Promoter: A Web-Based Tool for Boosting the Promotion of Clinical Research Through Social Media
- PMID: 27357424
- PMCID: PMC4945821
- DOI: 10.2196/jmir.4726
Trial Promoter: A Web-Based Tool for Boosting the Promotion of Clinical Research Through Social Media
Abstract
Background: Scarce information about clinical research, in particular clinical trials, is among the top reasons why potential participants do not take part in clinical studies. Without volunteers, on the other hand, clinical research and the development of novel approaches to preventing, diagnosing, and treating disease are impossible. Promising digital options such as social media have the potential to work alongside traditional methods to boost the promotion of clinical research. However, investigators and research institutions are challenged to leverage these innovations while saving time and resources.
Objective: To develop and test the efficiency of a Web-based tool that automates the generation and distribution of user-friendly social media messages about clinical trials.
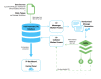
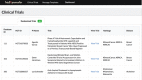
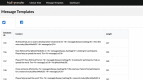
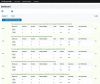
Methods: Trial Promoter is developed in Ruby on Rails, HTML, cascading style sheet (CSS), and JavaScript. In order to test the tool and the correctness of the generated messages, clinical trials (n=46) were randomized into social media messages and distributed via the microblogging social media platform Twitter and the social network Facebook. The percent correct was calculated to determine the probability with which Trial Promoter generates accurate messages.
Results: During a 10-week testing phase, Trial Promoter automatically generated and published 525 user-friendly social media messages on Twitter and Facebook. On average, Trial Promoter correctly used the message templates and substituted the message parameters (text, URLs, and disease hashtags) 97.7% of the time (1563/1600).
Conclusions: Trial Promoter may serve as a promising tool to render clinical trial promotion more efficient while requiring limited resources. It supports the distribution of any research or other types of content. The Trial Promoter code and installation instructions are freely available online.
Keywords: Facebook; Internet; Twitter; algorithm; automation; clinical trial; communication; online; patient; recruitment; social media; social network.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

Similar articles
-
A Software Tool Aimed at Automating the Generation, Distribution, and Assessment of Social Media Messages for Health Promotion and Education Research.JMIR Public Health Surveill. 2019 May 7;5(2):e11263. doi: 10.2196/11263. JMIR Public Health Surveill. 2019. PMID: 31066708 Free PMC article.
-
General Audience Engagement With Antismoking Public Health Messages Across Multiple Social Media Sites: Comparative Analysis.JMIR Public Health Surveill. 2021 Feb 19;7(2):e24429. doi: 10.2196/24429. JMIR Public Health Surveill. 2021. PMID: 33605890 Free PMC article.
-
A novel approach to conducting clinical trials in the community setting: utilizing patient-driven platforms and social media to drive web-based patient recruitment.BMC Med Res Methodol. 2020 Mar 13;20(1):58. doi: 10.1186/s12874-020-00926-y. BMC Med Res Methodol. 2020. PMID: 32169041 Free PMC article.
-
New media and tobacco control.Tob Control. 2012 Mar;21(2):139-44. doi: 10.1136/tobaccocontrol-2011-050193. Tob Control. 2012. PMID: 22345236 Review.
-
Social Media and Web Presence for Patients and Professionals: Evolving Trends and Implications for Practice.PM R. 2017 May;9(5S):S98-S105. doi: 10.1016/j.pmrj.2017.02.012. PM R. 2017. PMID: 28527508 Review.
Cited by
-
The Use of Social Media in Pediatric Urology-Forging New Paths or Crossing Boundaries?Curr Urol Rep. 2019 Oct 16;20(11):72. doi: 10.1007/s11934-019-0928-y. Curr Urol Rep. 2019. PMID: 31620926 Review.
-
Towards an Architecture to Guarantee Both Data Privacy and Utility in the First Phases of Digital Clinical Trials.Sensors (Basel). 2018 Nov 28;18(12):4175. doi: 10.3390/s18124175. Sensors (Basel). 2018. PMID: 30487435 Free PMC article.
-
Surveying Adolescents During a Pandemic: Comparison of Adolescents Recruited via Social Media vs. Schools.Prev Sci. 2023 Dec 1. doi: 10.1007/s11121-023-01621-2. Online ahead of print. Prev Sci. 2023. PMID: 38038891
References
-
- Public Engagement and Clinical Trials: New Models and Disruptive Technologies: Workshop Summary. Washington, DC: National Academies Press (US); 2012. - PubMed
-
- The Center for Information & Study on Clinical Research Participation. 2013. [2016-05-26]. Report on clinical trial information seekers. Perceptions and insights study https://www.ciscrp.org/
-
- The Center for Information & Study on Clinical Research Participation. 2015. [2016-05-26]. Report on the decision to participate. Perceptions and insights study https://www.ciscrp.org/
-
- Taylor H, Leitman R. Misconceptions and lack of awareness greatly reduce recruitment for cancer clinical trials. Harris Interactive: Health Care News. 2001;1(3):1–3.
-
- Fox S, Jones S. Pew Research Center. [2016-02-18]. The Social Life of Health Information http://www.pewinternet.org/files/old-media/Files/Reports/2009/PIP_Health... .
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

