Pironetin reacts covalently with cysteine-316 of α-tubulin to destabilize microtubule
- PMID: 27357539
- PMCID: PMC4931326
- DOI: 10.1038/ncomms12103
Pironetin reacts covalently with cysteine-316 of α-tubulin to destabilize microtubule
Abstract
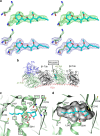
Molecules that alter the normal dynamics of microtubule assembly and disassembly include many anticancer drugs in clinical use. So far all such therapeutics target β-tubulin, and structural biology has explained the basis of their action and permitted design of new drugs. However, by shifting the profile of β-tubulin isoforms, cancer cells become resistant to treatment. Compounds that bind to α-tubulin are less well characterized and unexploited. The natural product pironetin is known to bind to α-tubulin and is a potent inhibitor of microtubule polymerization. Previous reports had identified that pironetin reacts with lysine-352 residue however analogues designed on this model had much lower potency, which was difficult to explain, hindering further development. We report crystallographic and mass spectrometric data that reveal that pironetin forms a covalent bond to cysteine-316 in α-tubulin via a Michael addition reaction. These data provide a basis for the rational design of α-tubulin targeting chemotherapeutics.
Figures




References
-
- Alberts B. et al. Molecular Biology of the Cell Garland Science (2008).
-
- Kupchan S. M. et al. Maytansine, a novel antileukemic ansa macrolide from Maytenus ovatus. J. Am. Chem. Soc. 94, 1354–1356 (1972). - PubMed
-
- Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer 10, 194–204 (2010). - PubMed
-
- Nogales E., Wolf S. G., Khan I. A., Luduena R. F. & Downing K. H. Structure of tubulin at 6.5 Å and location of the taxol-binding site. Nature 375, 424–427 (1995). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

