Adenoviral Delivery of Tumor Necrosis Factor-α and Interleukin-2 Enables Successful Adoptive Cell Therapy of Immunosuppressive Melanoma
- PMID: 27357626
- PMCID: PMC5023385
- DOI: 10.1038/mt.2016.137
Adenoviral Delivery of Tumor Necrosis Factor-α and Interleukin-2 Enables Successful Adoptive Cell Therapy of Immunosuppressive Melanoma
Erratum in
-
Corrigendum to "Adenoviral Delivery of Tumor Necrosis Factor-α and Interleukin-2 Enables Successful Adoptive Cell Therapy of Immunosuppressive Melanoma".Mol Ther. 2016 Nov;24(11):2033. doi: 10.1038/mt.2016.196. Mol Ther. 2016. PMID: 27916990 Free PMC article. No abstract available.
Abstract
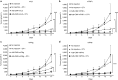
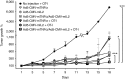
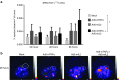
Adoptive T-cell transfer is a promising treatment approach for metastatic cancer, but efficacy in solid tumors has only been achieved with toxic pre- and postconditioning regimens. Thus, adoptive T-cell therapies would benefit from complementary modalities that enable their full potential without excessive toxicity. We aimed to improve the efficacy and safety of adoptive T-cell transfer by using adenoviral vectors for direct delivery of immunomodulatory murine cytokines into B16.OVA melanoma tumors with concomitant T-cell receptor transgenic OT-I T-cell transfer. Armed adenoviruses expressed high local and low systemic levels of cytokine when injected into B16.OVA tumors, suggesting safety of virus-mediated cytokine delivery. Antitumor efficacy was significantly enhanced with adenoviruses coding for murine interleukin-2 (mIL-2) and tumor necrosis factor-α (mTNFα) when compared with T-cell transfer alone or viruses alone. Further improvement in efficacy was achieved with a triple combination of mIL-2, mTNFα, and OT-I T-cells. Mechanistic studies suggest that mIL-2 has an important role in activating T-cells at the tumor, while mTNFα induces chemokine expression. Furthermore, adenovirus treatments enhanced tumor-infiltration of OT-I T-cells as demonstrated by SPECT/CT imaging of (111)In-labeled cells. Our results suggest the utility of cytokine-coding adenoviruses for improving the efficacy of adoptive T-cell therapies.
Figures


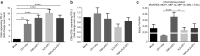
References
-
- June, CH, Riddell, SR and Schumacher, TN (2015). Adoptive cellular therapy: a race to the finish line. Sci Transl Med 7: 280ps7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

