Temporal and intrinsic factors of rifampicin tolerance in mycobacteria
- PMID: 27357669
- PMCID: PMC4961206
- DOI: 10.1073/pnas.1600372113
Temporal and intrinsic factors of rifampicin tolerance in mycobacteria
Abstract
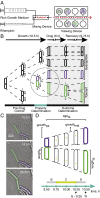
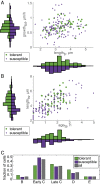
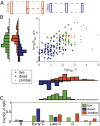
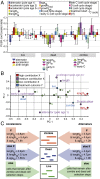
Mycobacteria grow and divide asymmetrically, creating variability in growth pole age, growth properties, and antibiotic susceptibilities. Here, we investigate the importance of growth pole age and other growth properties in determining the spectrum of responses of Mycobacterium smegmatis to challenge with rifampicin. We used a combination of live-cell microscopy and modeling to prospectively identify subpopulations with altered rifampicin susceptibility. We found two subpopulations that had increased susceptibility. At the initiation of treatment, susceptible cells were either small and at early stages of the cell cycle, or large and in later stages of their cell cycle. In contrast to this temporal window of susceptibility, tolerance was associated with factors inherited at division: long birth length and mature growth poles. Thus, rifampicin response is complex and due to a combination of differences established from both asymmetric division and the timing of treatment relative to cell birth.
Keywords: antibiotic susceptibility; cell biology; mathematical modeling; mycobacteria; single cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
Bacterial pathogenesis: Mycobacteria that are long in the tooth.Nat Rev Microbiol. 2016 Aug;14(8):474-5. doi: 10.1038/nrmicro.2016.106. Epub 2016 Jul 11. Nat Rev Microbiol. 2016. PMID: 27396564 No abstract available.
Similar articles
-
Clinically relevant mutations in mycobacterial LepA cause rifampicin-specific phenotypic resistance.Sci Rep. 2020 May 21;10(1):8402. doi: 10.1038/s41598-020-65308-2. Sci Rep. 2020. PMID: 32439911 Free PMC article.
-
Growth and division mechanisms by which genetic resisters emerge from the rifampicin-surviving population of differentially antibiotic-susceptible mycobacterial subpopulations.Int J Mycobacteriol. 2022 Jul-Sep;11(3):273-286. doi: 10.4103/ijmy.ijmy_88_22. Int J Mycobacteriol. 2022. PMID: 36260446
-
Asymmetry and aging of mycobacterial cells lead to variable growth and antibiotic susceptibility.Science. 2012 Jan 6;335(6064):100-4. doi: 10.1126/science.1216166. Epub 2011 Dec 15. Science. 2012. PMID: 22174129 Free PMC article.
-
Resistance to rifampicin: a review.J Antibiot (Tokyo). 2014 Sep;67(9):625-30. doi: 10.1038/ja.2014.107. Epub 2014 Aug 13. J Antibiot (Tokyo). 2014. PMID: 25118103 Review.
-
Mycobacterial Growth.Cold Spring Harb Perspect Med. 2015 May 8;5(10):a021097. doi: 10.1101/cshperspect.a021097. Cold Spring Harb Perspect Med. 2015. PMID: 25957314 Free PMC article. Review.
Cited by
-
A Parallel Adder Coordinates Mycobacterial Cell-Cycle Progression and Cell-Size Homeostasis in the Context of Asymmetric Growth and Organization.Curr Biol. 2017 Nov 6;27(21):3367-3374.e7. doi: 10.1016/j.cub.2017.09.046. Epub 2017 Oct 26. Curr Biol. 2017. PMID: 29107550 Free PMC article.
-
Mycobacterial Cultures Contain Cell Size and Density Specific Sub-populations of Cells with Significant Differential Susceptibility to Antibiotics, Oxidative and Nitrite Stress.Front Microbiol. 2017 Mar 21;8:463. doi: 10.3389/fmicb.2017.00463. eCollection 2017. Front Microbiol. 2017. PMID: 28377757 Free PMC article.
-
Morphological profiling of tubercle bacilli identifies drug pathways of action.Proc Natl Acad Sci U S A. 2020 Aug 4;117(31):18744-18753. doi: 10.1073/pnas.2002738117. Epub 2020 Jul 17. Proc Natl Acad Sci U S A. 2020. PMID: 32680963 Free PMC article.
-
Rifampicin can induce antibiotic tolerance in mycobacteria via paradoxical changes in rpoB transcription.Nat Commun. 2018 Oct 11;9(1):4218. doi: 10.1038/s41467-018-06667-3. Nat Commun. 2018. PMID: 30310059 Free PMC article.
-
Fluorescent Mycobacterium tuberculosis reporters: illuminating host-pathogen interactions.Pathog Dis. 2018 Apr 1;76(3):fty017. doi: 10.1093/femspd/fty017. Pathog Dis. 2018. PMID: 29718182 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

