Gpr161 anchoring of PKA consolidates GPCR and cAMP signaling
- PMID: 27357676
- PMCID: PMC4948347
- DOI: 10.1073/pnas.1608061113
Gpr161 anchoring of PKA consolidates GPCR and cAMP signaling
Abstract
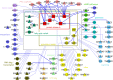
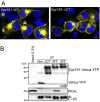
Scaffolding proteins organize the information flow from activated G protein-coupled receptors (GPCRs) to intracellular effector cascades both spatially and temporally. By this means, signaling scaffolds, such as A-kinase anchoring proteins (AKAPs), compartmentalize kinase activity and ensure substrate selectivity. Using a phosphoproteomics approach we identified a physical and functional connection between protein kinase A (PKA) and Gpr161 (an orphan GPCR) signaling. We show that Gpr161 functions as a selective high-affinity AKAP for type I PKA regulatory subunits (RI). Using cell-based reporters to map protein-protein interactions, we discovered that RI binds directly and selectively to a hydrophobic protein-protein interaction interface in the cytoplasmic carboxyl-terminal tail of Gpr161. Furthermore, our data demonstrate that a binary complex between Gpr161 and RI promotes the compartmentalization of Gpr161 to the plasma membrane. Moreover, we show that Gpr161, functioning as an AKAP, recruits PKA RI to primary cilia in zebrafish embryos. We also show that Gpr161 is a target of PKA phosphorylation, and that mutation of the PKA phosphorylation site affects ciliary receptor localization. Thus, we propose that Gpr161 is itself an AKAP and that the cAMP-sensing Gpr161:PKA complex acts as cilium-compartmentalized signalosome, a concept that now needs to be considered in the analyzing, interpreting, and pharmaceutical targeting of PKA-associated functions.
Keywords: interaction network; molecular interactions; phosphorylation; primary cilium; scaffolding function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
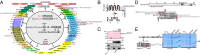








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

