Basal forebrain projections to the lateral habenula modulate aggression reward
- PMID: 27357796
- PMCID: PMC4930107
- DOI: 10.1038/nature18601
Basal forebrain projections to the lateral habenula modulate aggression reward
Abstract
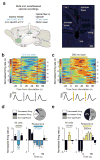
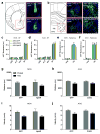
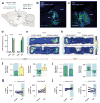
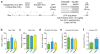
Maladaptive aggressive behaviour is associated with a number of neuropsychiatric disorders and is thought to result partly from the inappropriate activation of brain reward systems in response to aggressive or violent social stimuli. Nuclei within the ventromedial hypothalamus, extended amygdala and limbic circuits are known to encode initiation of aggression; however, little is known about the neural mechanisms that directly modulate the motivational component of aggressive behaviour. Here we established a mouse model to measure the valence of aggressive inter-male social interaction with a smaller subordinate intruder as reinforcement for the development of conditioned place preference (CPP). Aggressors develop a CPP, whereas non-aggressors develop a conditioned place aversion to the intruder-paired context. Furthermore, we identify a functional GABAergic projection from the basal forebrain (BF) to the lateral habenula (lHb) that bi-directionally controls the valence of aggressive interactions. Circuit-specific silencing of GABAergic BF-lHb terminals of aggressors with halorhodopsin (NpHR3.0) increases lHb neuronal firing and abolishes CPP to the intruder-paired context. Activation of GABAergic BF-lHb terminals of non-aggressors with channelrhodopsin (ChR2) decreases lHb neuronal firing and promotes CPP to the intruder-paired context. Finally, we show that altering inhibitory transmission at BF-lHb terminals does not control the initiation of aggressive behaviour. These results demonstrate that the BF-lHb circuit has a critical role in regulating the valence of inter-male aggressive behaviour and provide novel mechanistic insight into the neural circuits modulating aggression reward processing.
Conflict of interest statement
The authors wish to declare no competing financial interests.
Figures








References
-
- Wasman M, Flynn JP. Directed attack elicited from hypothalamus. Arch Neurol. 1962;6:220–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- FI2 GM117583/GM/NIGMS NIH HHS/United States
- R01 MH104559/MH/NIMH NIH HHS/United States
- F31 MH105217/MH/NIMH NIH HHS/United States
- 1FI2GM117583-01/GM/NIGMS NIH HHS/United States
- P50 AT008661/AT/NCCIH NIH HHS/United States
- R01 MH073689/MH/NIMH NIH HHS/United States
- T32 MH096678/MH/NIMH NIH HHS/United States
- R01 MH092306/MH/NIMH NIH HHS/United States
- R21 MH112081/MH/NIMH NIH HHS/United States
- T32 MH087004/MH/NIMH NIH HHS/United States
- F30 MH100835/MH/NIMH NIH HHS/United States
- P50 AT008661-01/AT/NCCIH NIH HHS/United States
- P50 MH096890/MH/NIMH NIH HHS/United States
- R01 MH090264/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

