Coordinating cardiomyocyte interactions to direct ventricular chamber morphogenesis
- PMID: 27357797
- PMCID: PMC5330678
- DOI: 10.1038/nature18310
Coordinating cardiomyocyte interactions to direct ventricular chamber morphogenesis
Abstract
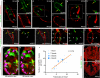
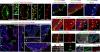
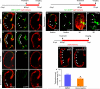
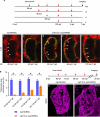
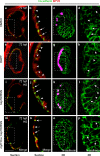
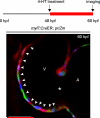
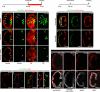
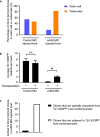
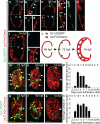
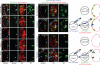
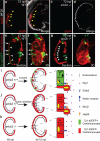
Many organs are composed of complex tissue walls that are structurally organized to optimize organ function. In particular, the ventricular myocardial wall of the heart comprises an outer compact layer that concentrically encircles the ridge-like inner trabecular layer. Although disruption in the morphogenesis of this myocardial wall can lead to various forms of congenital heart disease and non-compaction cardiomyopathies, it remains unclear how embryonic cardiomyocytes assemble to form ventricular wall layers of appropriate spatial dimensions and myocardial mass. Here we use advanced genetic and imaging tools in zebrafish to reveal an interplay between myocardial Notch and Erbb2 signalling that directs the spatial allocation of myocardial cells to their proper morphological positions in the ventricular wall. Although previous studies have shown that endocardial Notch signalling non-cell-autonomously promotes myocardial trabeculation through Erbb2 and bone morphogenetic protein (BMP) signalling, we discover that distinct ventricular cardiomyocyte clusters exhibit myocardial Notch activity that cell-autonomously inhibits Erbb2 signalling and prevents cardiomyocyte sprouting and trabeculation. Myocardial-specific Notch inactivation leads to ventricles of reduced size and increased wall thickness because of excessive trabeculae, whereas widespread myocardial Notch activity results in ventricles of increased size with a single-cell-thick wall but no trabeculae. Notably, this myocardial Notch signalling is activated non-cell-autonomously by neighbouring Erbb2-activated cardiomyocytes that sprout and form nascent trabeculae. Thus, these findings support an interactive cellular feedback process that guides the assembly of cardiomyocytes to morphologically create the ventricular myocardial wall and more broadly provide insight into the cellular dynamics of how diverse cell lineages organize to create form.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

