Mitochondrial health, the epigenome and healthspan
- PMID: 27358026
- PMCID: PMC5066813
- DOI: 10.1042/CS20160002
Mitochondrial health, the epigenome and healthspan
Abstract
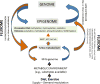
Food nutrients and metabolic supply-demand dynamics constitute environmental factors that interact with our genome influencing health and disease states. These gene-environment interactions converge at the metabolic-epigenome-genome axis to regulate gene expression and phenotypic outcomes. Mounting evidence indicates that nutrients and lifestyle strongly influence genome-metabolic functional interactions determining disease via altered epigenetic regulation. The mitochondrial network is a central player of the metabolic-epigenome-genome axis, regulating the level of key metabolites [NAD(+), AcCoA (acetyl CoA), ATP] acting as substrates/cofactors for acetyl transferases, kinases (e.g. protein kinase A) and deacetylases (e.g. sirtuins, SIRTs). The chromatin, an assembly of DNA and nucleoproteins, regulates the transcriptional process, acting at the epigenomic interface between metabolism and the genome. Within this framework, we review existing evidence showing that preservation of mitochondrial network function is directly involved in decreasing the rate of damage accumulation thus slowing aging and improving healthspan.
Keywords: acetylation; adipose tissue; aging; autophagy; biogenesis; caloric restriction; cardiovascular disease; chromatin; diet; epigenetic modification; epigenetics; histones; mitochondria; mitochondrial fusion–fission; mitophagy.
© 2016 The Author(s). published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
All Authors declare no conflict of interest.
Figures



References
-
- Finkel T. Radical medicine: treating ageing to cure disease. Nat Rev Mol Cell Biol. 2005;6:971–976. - PubMed
-
- Kembro JM, Cortassa S, Aon MA. In: Mitochondrial ROS and arrhythmias In Systems Biology of free radicals and anti-oxidants. Laher I, editor. Springer-Verlag; 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

