Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group
- PMID: 27358379
- PMCID: PMC4999563
- DOI: 10.1093/annonc/mdw261
Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group
Abstract
Background: To explore the impact of KRAS, NRAS and BRAF mutations as well as KRAS mutation variants in patients with metastatic colorectal cancer (mCRC) receiving first-line therapy.
Patients and methods: A total of 1239 patients from five randomized trials (FIRE-1, FIRE-3, AIOKRK0207, AIOKRK0604, RO91) were included into the analysis. Outcome was evaluated by the Kaplan-Meier method, log-rank tests and Cox models.
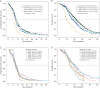
Results: In 664 tumors, no mutation was detected, 462 tumors were diagnosed with KRAS-, 39 patients with NRAS- and 74 patients with BRAF-mutation. Mutations in KRAS were associated with inferior progression-free survival (PFS) and overall survival (OS) [multivariate hazard ratio (HR) for PFS: 1.20 (1.02-1.42), P = 0.03; multivariate HR for OS: 1.41 (1.17-1.70), P < 0.001]. BRAF mutation was also associated with inferior PFS [multivariate HR: 2.19 (1.59-3.02), P < 0.001] and OS [multivariate HR: 2.99 (2.10-4.25), P < 0.001]. Among specific KRAS mutation variants, the KRAS G12C-variant (n = 28) correlated with inferior OS compared with unmutated tumors [multivariate HR 2.26 (1.25-4.1), P = 0.001]. A similar trend for OS was seen in the KRAS G13D-variant [n = 71, multivariate HR 1.46 (0.96-2.22), P = 0.10]. More frequent KRAS exon 2 variants like G12D [n = 152, multivariate HR 1.17 (0.86-1.6), P = 0.81] and G12V [n = 92, multivariate HR 1.27 (0.87-1.86), P = 0.57] did not have significant impact on OS.
Conclusion: Mutations in KRAS and BRAF were associated with inferior PFS and OS of mCRC patients compared with patients with non-mutated tumors. KRAS exon 2 mutation variants were associated with heterogeneous outcome compared with unmutated tumors with KRAS G12C and G13D (trend) being associated with rather poor survival.
Keywords: BRAF; RAS; colorectal cancer; mutation; prognostic factor.
© The Author 2016. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures



References
-
- Douillard JY, Oliner KS, Siena S et al. . Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013; 369: 1023–1034. - PubMed
-
- Heinemann V, von Weikersthal LF, Decker T et al. . FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15: 1065–1075. - PubMed
-
- Van Cutsem E, Lenz HJ, Kohne CH et al. . Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015; 33: 692–700. - PubMed
-
- Bazan V, Migliavacca M, Zanna I et al. . Specific codon 13 K-ras mutations are predictive of clinical outcome in colorectal cancer patients, whereas codon 12 K-ras mutations are associated with mucinous histotype. Ann Oncol 2002; 13: 1438–1446. - PubMed
-
- Cremolini C, Loupakis F, Antoniotti C et al. . FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015; 16: 1306–1315. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

