ERK1/2 activation in human taste bud cells regulates fatty acid signaling and gustatory perception of fat in mice and humans
- PMID: 27358389
- PMCID: PMC5024696
- DOI: 10.1096/fj.201600422R
ERK1/2 activation in human taste bud cells regulates fatty acid signaling and gustatory perception of fat in mice and humans
Abstract
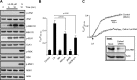
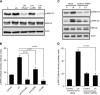
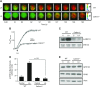
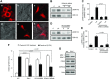
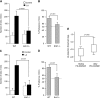
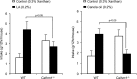
Obesity is a major public health problem. An in-depth knowledge of the molecular mechanisms of oro-sensory detection of dietary lipids may help fight it. Humans and rodents can detect fatty acids via lipido-receptors, such as CD36 and GPR120. We studied the implication of the MAPK pathways, in particular, ERK1/2, in the gustatory detection of fatty acids. Linoleic acid, a dietary fatty acid, induced via CD36 the phosphorylation of MEK1/2-ERK1/2-ETS-like transcription factor-1 cascade, which requires Fyn-Src kinase and lipid rafts in human taste bud cells (TBCs). ERK1/2 cascade was activated by Ca2+ signaling via opening of the calcium-homeostasis modulator-1 (CALHM1) channel. Furthermore, fatty acid-evoked Ca2+ signaling and ERK1/2 phosphorylation were decreased in both human TBCs after small interfering RNA knockdown of CALHM1 channel and in TBCs from Calhm1-/- mice. Targeted knockdown of ERK1/2 by small interfering RNA or PD0325901 (MEK1/2 inhibitor) in the tongue and genetic ablation of Erk1 or Calhm1 genes impaired preference for dietary fat in mice. Lingual inhibition of ERK1/2 in healthy volunteers also decreased orogustatory sensitivity for linoleic acid. Our data demonstrate that ERK1/2-MAPK cascade is regulated by the opening of CALHM1 Ca2+ channel in TBCs to modulate orogustatory detection of dietary lipids in mice and humans.-Subramaniam, S., Ozdener, M. H., Abdoul-Azize, S., Saito, K., Malik, B., Maquart, G., Hashimoto, T., Marambaud, P., Aribi, M., Tordoff, M. G., Besnard, P., Khan, N. A. ERK1/2 activation in human taste bud cells regulates fatty acid signaling and gustatory perception of fat in mice and humans.
Keywords: CALHM1; MAPK; lipids.
© FASEB.
Figures


References
-
- Gurevich-Panigrahi T., Panigrahi S., Wiechec E., Los M. (2009) Obesity: pathophysiology and clinical management. Curr. Med. Chem. 16, 506–521 - PubMed
-
- Mela D. J., Sacchetti D. A. (1991) Sensory preferences for fats: relationships with diet and body composition. Am. J. Clin. Nutr. 53, 908–915 - PubMed
-
- Besnard P., Passilly-Degrace P., Khan N. A. (2016) Taste of fat: a sixth taste modality? Physiol. Rev. 96, 151–176 - PubMed
-
- Gilbertson T. A., Khan N. A. (2014) Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog. Lipid Res. 53, 82–92 - PubMed
-
- Chalé-Rush A., Burgess J. R., Mattes R. D. (2007) Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem. Senses 32, 423–431 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

