Gadd45a Protein Promotes Skeletal Muscle Atrophy by Forming a Complex with the Protein Kinase MEKK4
- PMID: 27358404
- PMCID: PMC5016147
- DOI: 10.1074/jbc.M116.740308
Gadd45a Protein Promotes Skeletal Muscle Atrophy by Forming a Complex with the Protein Kinase MEKK4
Abstract
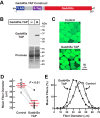
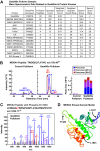
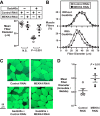
Skeletal muscle atrophy is a serious and highly prevalent condition that remains poorly understood at the molecular level. Previous work found that skeletal muscle atrophy involves an increase in skeletal muscle Gadd45a expression, which is necessary and sufficient for skeletal muscle fiber atrophy. However, the direct mechanism by which Gadd45a promotes skeletal muscle atrophy was unknown. To address this question, we biochemically isolated skeletal muscle proteins that associate with Gadd45a as it induces atrophy in mouse skeletal muscle fibers in vivo We found that Gadd45a interacts with multiple proteins in skeletal muscle fibers, including, most prominently, MEKK4, a mitogen-activated protein kinase kinase kinase that was not previously known to play a role in skeletal muscle atrophy. Furthermore, we found that, by forming a complex with MEKK4 in skeletal muscle fibers, Gadd45a increases MEKK4 protein kinase activity, which is both sufficient to induce skeletal muscle fiber atrophy and required for Gadd45a-mediated skeletal muscle fiber atrophy. Together, these results identify a direct biochemical mechanism by which Gadd45a induces skeletal muscle atrophy and provide new insight into the way that skeletal muscle atrophy occurs at the molecular level.
Keywords: Gadd45a; MEKK4; aging; mass spectrometry (MS); muscle; muscle atrophy; skeletal muscle; skeletal muscle metabolism.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
Skeletal muscle denervation causes skeletal muscle atrophy through a pathway that involves both Gadd45a and HDAC4.Am J Physiol Endocrinol Metab. 2013 Oct 1;305(7):E907-15. doi: 10.1152/ajpendo.00380.2013. Epub 2013 Aug 13. Am J Physiol Endocrinol Metab. 2013. PMID: 23941879 Free PMC article.
-
Stress-induced skeletal muscle Gadd45a expression reprograms myonuclei and causes muscle atrophy.J Biol Chem. 2012 Aug 10;287(33):27290-301. doi: 10.1074/jbc.M112.374777. Epub 2012 Jun 12. J Biol Chem. 2012. PMID: 22692209 Free PMC article.
-
Role of ATF4 in skeletal muscle atrophy.Curr Opin Clin Nutr Metab Care. 2017 May;20(3):164-168. doi: 10.1097/MCO.0000000000000362. Curr Opin Clin Nutr Metab Care. 2017. PMID: 28376050 Review.
-
Activating transcription factor 4 (ATF4) promotes skeletal muscle atrophy by forming a heterodimer with the transcriptional regulator C/EBPβ.J Biol Chem. 2020 Feb 28;295(9):2787-2803. doi: 10.1074/jbc.RA119.012095. Epub 2020 Jan 17. J Biol Chem. 2020. PMID: 31953319 Free PMC article.
-
Gadd45a, a p53- and BRCA1-regulated stress protein, in cellular response to DNA damage.Mutat Res. 2005 Jan 6;569(1-2):133-43. doi: 10.1016/j.mrfmmm.2004.06.055. Mutat Res. 2005. PMID: 15603758 Review.
Cited by
-
FoxO Transcription Factors Are Critical Regulators of Diabetes-Related Muscle Atrophy.Diabetes. 2019 Mar;68(3):556-570. doi: 10.2337/db18-0416. Epub 2018 Dec 6. Diabetes. 2019. PMID: 30523026 Free PMC article.
-
An investigation of p53 in skeletal muscle aging.J Appl Physiol (1985). 2019 Oct 1;127(4):1075-1084. doi: 10.1152/japplphysiol.00363.2019. Epub 2019 Aug 29. J Appl Physiol (1985). 2019. PMID: 31465716 Free PMC article.
-
Biology of Activating Transcription Factor 4 (ATF4) and Its Role in Skeletal Muscle Atrophy.J Nutr. 2022 Apr 1;152(4):926-938. doi: 10.1093/jn/nxab440. J Nutr. 2022. PMID: 34958390 Free PMC article. Review.
-
Muscle Atrophy Induced by Mechanical Unloading: Mechanisms and Potential Countermeasures.Front Physiol. 2018 Mar 20;9:235. doi: 10.3389/fphys.2018.00235. eCollection 2018. Front Physiol. 2018. PMID: 29615929 Free PMC article. Review.
-
GADD45A regulates subcutaneous fat deposition and lipid metabolism by interacting with Stat1.BMC Biol. 2023 Oct 9;21(1):212. doi: 10.1186/s12915-023-01713-z. BMC Biol. 2023. PMID: 37807064 Free PMC article.
References
-
- Hoffman B., and Liebermann D. A. (2009) Gadd45 modulation of intrinsic and extrinsic stress responses in myeloid cells. J. Cell. Physiol. 218, 26–31 - PubMed
-
- Salvador J. M., Brown-Clay J. D., and Fornace A. J. Jr. (2013) Gadd45 in stress signaling, cell cycle control, and apoptosis. Adv. Exp. Med. Biol. 793, 1–19 - PubMed
-
- Lee C. K., Klopp R. G., Weindruch R., and Prolla T. A. (1999) Gene expression profile of aging and its retardation by caloric restriction. Science 285, 1390–1393 - PubMed
-
- Welle S., Brooks A. I., Delehanty J. M., Needler N., and Thornton C. A. (2003) Gene expression profile of aging in human muscle. Physiol. Genomics 14, 149–159 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

