Development and Validation of an Empirical Dietary Inflammatory Index
- PMID: 27358416
- PMCID: PMC4958288
- DOI: 10.3945/jn.115.228718
Development and Validation of an Empirical Dietary Inflammatory Index
Abstract
Background: Knowledge on specific biological pathways mediating disease occurrence (e.g., inflammation) may be utilized to construct hypotheses-driven dietary patterns that take advantage of current evidence on disease-related hypotheses and the statistical methods of a posteriori patterns.
Objective: We developed and validated an empirical dietary inflammatory index (EDII) based on food groups.
Methods: We entered 39 pre-defined food groups in reduced rank regression models followed by stepwise linear regression analyses in the Nurses' Health Study (NHS, n = 5230) to identify a dietary pattern most predictive of 3 plasma inflammatory markers: interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor α receptor 2 (TNFαR2). We evaluated the construct validity of the EDII in 2 independent samples from NHS-II (n = 1002) and Health Professionals Follow-up Study (HPFS, n = 2632) using multivariable-adjusted linear regression models to examine how well the EDII predicted concentrations of IL-6, CRP, TNFαR2, adiponectin, and an overall inflammatory marker score combining all biomarkers.
Results: The EDII is the weighted sum of 18 food groups; 9 are anti-inflammatory and 9 proinflammatory. In NHS-II and HPFS, the EDII significantly predicted concentrations of all biomarkers. For example, the relative concentrations comparing extreme EDII quintiles in NHS-II were: adiponectin, 0.88 (95% CI, 0.80, 0.96), P-trend = 0.003; and CRP, 1.52 (95% CI, 1.18, 1.97), P-trend = 0.002. Corresponding associations in HPFS were: 0.87 (95% CI, 0.82, 0.92), P-trend < 0.0001; and 1.23 (95% CI, 1.09, 1.40), P-trend = 0.002.
Conclusion: The EDII represents, to our knowledge, a novel, hypothesis-driven, empirically derived dietary pattern that assesses diet quality based on its inflammatory potential. Its strong construct validity in independent samples of women and men indicates its usefulness in assessing the inflammatory potential of whole diets. Additionally, the EDII may be calculated in a standardized and reproducible manner across different populations thus circumventing a major limitation of dietary patterns derived from the same study in which they are applied.
Keywords: dietary inflammatory potential; dietary patterns; hypothesis-driven; inflammation; inflammatory markers.
© 2016 American Society for Nutrition.
Conflict of interest statement
Author disclosures: FK Tabung, SA Smith-Warner, JE Chavarro, K Wu, CS Fuchs, FB Hu, AT Chan, WC Willet, and EL Giovannucci, no conflicts of interest.
Figures
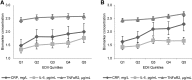
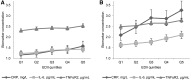
Similar articles
-
An Empirical Dietary Inflammatory Pattern Score Enhances Prediction of Circulating Inflammatory Biomarkers in Adults.J Nutr. 2017 Aug;147(8):1567-1577. doi: 10.3945/jn.117.248377. Epub 2017 Jun 28. J Nutr. 2017. PMID: 28659407 Free PMC article.
-
An Empirical Dietary Inflammatory Pattern Score Is Associated with Circulating Inflammatory Biomarkers in a Multi-Ethnic Population of Postmenopausal Women in the United States.J Nutr. 2018 May 1;148(5):771-780. doi: 10.1093/jn/nxy031. J Nutr. 2018. PMID: 29897561 Free PMC article. Clinical Trial.
-
Construct validation of the dietary inflammatory index among postmenopausal women.Ann Epidemiol. 2015 Jun;25(6):398-405. doi: 10.1016/j.annepidem.2015.03.009. Epub 2015 Mar 19. Ann Epidemiol. 2015. PMID: 25900255 Free PMC article.
-
Food-based indexes and their association with dietary inflammation.Adv Nutr. 2025 Apr;16(4):100400. doi: 10.1016/j.advnut.2025.100400. Epub 2025 Mar 4. Adv Nutr. 2025. PMID: 40043850 Free PMC article.
-
Association between dietary inflammatory index and inflammatory markers in the HELENA study.Mol Nutr Food Res. 2017 Jun;61(6):10.1002/mnfr.201600707. doi: 10.1002/mnfr.201600707. Epub 2017 Feb 22. Mol Nutr Food Res. 2017. PMID: 27981781 Free PMC article. Review.
Cited by
-
Diet in Rheumatoid Arthritis versus Systemic Lupus Erythematosus: Any Differences?Nutrients. 2021 Feb 27;13(3):772. doi: 10.3390/nu13030772. Nutrients. 2021. PMID: 33673487 Free PMC article. Review.
-
Empirical dietary inflammatory index and lifestyle inflammation score relationship with obesity: A population-based cross-sectional study.Food Sci Nutr. 2023 Sep 13;11(11):7341-7351. doi: 10.1002/fsn3.3660. eCollection 2023 Nov. Food Sci Nutr. 2023. PMID: 37970372 Free PMC article.
-
Red blood cell membrane trans fatty acid levels and risk of non-Hodgkin lymphoma: a prospective nested case-control study.Am J Clin Nutr. 2020 Dec 10;112(6):1576-1583. doi: 10.1093/ajcn/nqaa251. Am J Clin Nutr. 2020. PMID: 33022699 Free PMC article.
-
Proinflammatory Diet Is Associated With Increased Risk of Fecal Incontinence Among Older Women: Prospective Results From the Nurses' Health Study.Clin Gastroenterol Hepatol. 2023 Jun;21(6):1657-1659.e3. doi: 10.1016/j.cgh.2022.04.011. Epub 2022 Apr 30. Clin Gastroenterol Hepatol. 2023. PMID: 35504561 Free PMC article.
-
The Role of Diet and Gut Microbiota in Regulating Gastrointestinal and Inflammatory Disease.Front Immunol. 2022 Apr 5;13:866059. doi: 10.3389/fimmu.2022.866059. eCollection 2022. Front Immunol. 2022. PMID: 35450067 Free PMC article. Review.
References
-
- Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9. - PubMed
-
- Jacques PF, Tucker KL. Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr 2001;73:1–2. - PubMed
-
- World Cancer Research Fund and the American Institute for Cancer Research. Food, nutrition and prevention of cancer: a global perspective 2007. Washington (DC). Report No.: 978–0-9722522–2-5.
-
- Varraso R, Garcia-Aymerich J, Monier F, Le Moual N, De Batlle J, Miranda G, Pison C, Romieu I, Kauffmann F, Maccario J. Assessment of dietary patterns in nutritional epidemiology: principal component analysis compared with confirmatory factor analysis. Am J Clin Nutr 2012;96:1079–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

