Muscle IL1β Drives Ischemic Myalgia via ASIC3-Mediated Sensory Neuron Sensitization
- PMID: 27358445
- PMCID: PMC4926236
- DOI: 10.1523/JNEUROSCI.4582-15.2016
Muscle IL1β Drives Ischemic Myalgia via ASIC3-Mediated Sensory Neuron Sensitization
Abstract
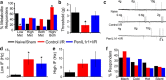
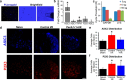
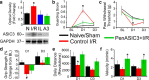
Musculoskeletal pain is a significantly common clinical complaint. Although it is known that muscles are quite sensitive to alterations in blood flow/oxygenation and a number of muscle pain disorders are based in problems of peripheral perfusion, the mechanisms by which ischemic-like conditions generate myalgia remain unclear. We found, using a multidisciplinary experimental approach, that ischemia and reperfusion injury (I/R) in male Swiss Webster mice altered ongoing and evoked pain-related behaviors in addition to activity levels through enhanced muscle interleukin-1 beta (IL1β)/IL1 receptor signaling to group III/IV muscle afferents. Peripheral sensitization depended on acid-sensing ion channels (ASICs) because treatment of sensory afferents in vitro with IL1β-upregulated ASIC3 in single cells, and nerve-specific knock-down of ASIC3 recapitulated the results of inhibiting the enhanced IL1β/IL1r1 signaling after I/R, which was also found to regulate afferent sensitization and pain-related behaviors. This suggests that targeting muscle IL1β signaling may be a potential analgesic therapy for ischemic myalgia.
Significance statement: Here, we have described a novel pathway whereby increased inflammation within the muscle tissue during ischemia/reperfusion injury sensitizes group III and IV muscle afferents via upregulation of acid-sensing ion channel 3 (ASIC3), leading not only to alterations in mechanical and chemical responsiveness in individual afferents, but also to pain-related behavioral changes. Furthermore, these I/R-induced changes can be prevented using an afferent-specific siRNA knock-down strategy targeting either ASIC3 or the upstream mediator of its expression, interleukin 1 receptor 1. Therefore, this knowledge may contribute to the development of alternative therapeutics for muscle pain and may be especially relevant to pain caused by issues of peripheral circulation, which is commonly observed in disorders such as complex regional pain syndrome, sickle cell anemia, or fibromyalgia.
Keywords: ASICs; behavior; cytokines; electrophysiology; siRNAs; signaling.
Copyright © 2016 the authors 0270-6474/16/366857-15$15.00/0.
Figures





Similar articles
-
Interleukin 1β inhibition contributes to the antinociceptive effects of voluntary exercise on ischemia/reperfusion-induced hypersensitivity.Pain. 2018 Feb;159(2):380-392. doi: 10.1097/j.pain.0000000000001094. Pain. 2018. PMID: 29112534 Free PMC article.
-
Upregulations of P2X(3) and ASIC3 involve in hyperalgesia induced by cisplatin administration in rats.Pain. 2010 May;149(2):393-405. doi: 10.1016/j.pain.2010.03.005. Epub 2010 Apr 8. Pain. 2010. PMID: 20378247
-
ASIC1 and ASIC3 play different roles in the development of Hyperalgesia after inflammatory muscle injury.J Pain. 2010 Mar;11(3):210-8. doi: 10.1016/j.jpain.2009.07.004. Epub 2009 Dec 16. J Pain. 2010. PMID: 20015700 Free PMC article.
-
Peripheral Mechanisms of Ischemic Myalgia.Front Cell Neurosci. 2017 Dec 22;11:419. doi: 10.3389/fncel.2017.00419. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29311839 Free PMC article. Review.
-
P2X3 receptors are transducers of sensory signals.Brain Res Bull. 2019 Sep;151:119-124. doi: 10.1016/j.brainresbull.2018.12.020. Epub 2019 Jan 17. Brain Res Bull. 2019. PMID: 30660716 Review.
Cited by
-
Early Life Nociception is Influenced by Peripheral Growth Hormone Signaling.J Neurosci. 2021 May 19;41(20):4410-4427. doi: 10.1523/JNEUROSCI.3081-20.2021. Epub 2021 Apr 22. J Neurosci. 2021. PMID: 33888610 Free PMC article.
-
Salient type 1 interleukin 1 receptor expression in peripheral non-immune cells.Sci Rep. 2018 Jan 15;8(1):723. doi: 10.1038/s41598-018-19248-7. Sci Rep. 2018. PMID: 29335509 Free PMC article.
-
Acid-sensing ion channels in sensory signaling.Am J Physiol Renal Physiol. 2020 Mar 1;318(3):F531-F543. doi: 10.1152/ajprenal.00546.2019. Epub 2020 Jan 27. Am J Physiol Renal Physiol. 2020. PMID: 31984789 Free PMC article. Review.
-
Chick Embryo: A Preclinical Model for Understanding Ischemia-Reperfusion Mechanism.Front Pharmacol. 2018 Sep 21;9:1034. doi: 10.3389/fphar.2018.01034. eCollection 2018. Front Pharmacol. 2018. PMID: 30298003 Free PMC article.
-
C-Jun N-Terminal Kinase Post-Translational Regulation of Pain-Related Acid-Sensing Ion Channels 1b and 3.J Neurosci. 2021 Oct 20;41(42):8673-8685. doi: 10.1523/JNEUROSCI.0570-21.2021. Epub 2021 Aug 11. J Neurosci. 2021. PMID: 34380759 Free PMC article.
References
-
- Benson CJ, McCleskey EW. ASICs function as lactic acid sensors during cardiac ischemia. In: Wang DH, editor. Molecular sensors for cardiovascular homeostasis. New York: Springer; 2007. pp. 32–50.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
