Efficacy and safety of gemcitabine plus erlotinib for locally advanced or metastatic pancreatic cancer: a systematic review and meta-analysis
- PMID: 27358556
- PMCID: PMC4912328
- DOI: 10.2147/DDDT.S105442
Efficacy and safety of gemcitabine plus erlotinib for locally advanced or metastatic pancreatic cancer: a systematic review and meta-analysis
Abstract
Background: Pancreatic cancer is considered as a chemoresistant neoplasm with extremely dismal prognosis. Gemcitabine is recommended as the standard agent for locally advanced or metastatic pancreatic cancer. A series of trials have been conducted to improve the outcome of advanced pancreatic cancer with other anticancer drugs in combination with gemcitabine. Unfortunately, the designers of the clinical trials failed to improve the poor prognosis of patients with advanced pancreatic cancer. Erlotinib was the first additional drug that improved the overall survival of patients with advanced pancreatic cancer with gemcitabine. We performed this systematic review and meta-analysis to explore the efficacy and safety of the combination of gemcitabine with erlotinib (GemErlo) for patients with advanced pancreatic cancer using the currently available evidence.
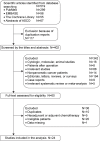
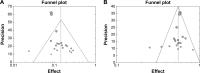
Methods: PubMed/MEDLINE, EMBASE, the Cochrane Library, and relevant abstracts of major conferences were comprehensively searched. Data results on objective response rate, disease control rate, and 1-year survival were pooled by using MetaAnalyst with a random-effects model. Results on progression-free survival and overall survival were only summarized descriptively.
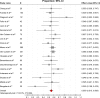
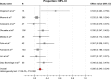
Results: A total of 24 studies with 1,742 patients with locally advanced or metastatic pancreatic cancer treated with GemErlo were included. Combined objective response rate was 14.4% (95% CI: 11.6%-17.7%), disease control rate was 55.0% (95% CI: 51.5%-58.5%), and 1-year survival rate was 28.5% (95% CI: 24.0%-33.4%). Progression-free survival ranged from 2.63 to 9.6 months, and overall survival varied from 6 to 10 months. As for the toxicity profile, the most common adverse events (AEs) were hematologic reactions, skin rash, and gastrointestinal reactions. Other severe AEs, which had low incidence, included treatment-induced death and interstitial lung disease.
Conclusion: Our study showed that GemErlo is associated with reasonable activity in treating patients with locally advanced or metastatic pancreatic cancer. Most of the AEs were tolerable, while some severe AEs needed careful detection.
Keywords: advanced pancreatic cancer; chemotherapy; meta-analysis; targeted agent.
Figures

References
-
- Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2009;20:197–209. - PubMed
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics. 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. - PubMed
-
- Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. BMJ. 2012;344:e2476. - PubMed
-
- Cascinu S, Graziano F, Catalano G. Chemotherapy for advanced pancreatic cancer: it may no longer be ignored. Ann Oncol. 1999;10:105–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

