BST2 Mediates Osteoblast Differentiation via the BMP2 Signaling Pathway in Human Alveolar-Derived Bone Marrow Stromal Cells
- PMID: 27359105
- PMCID: PMC4928849
- DOI: 10.1371/journal.pone.0158481
BST2 Mediates Osteoblast Differentiation via the BMP2 Signaling Pathway in Human Alveolar-Derived Bone Marrow Stromal Cells
Abstract
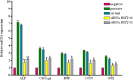
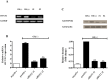
The molecular mechanisms controlling the differentiation of bone marrow stromal stem cells into osteoblasts remain largely unknown. In this study, we investigated whether bone marrow stromal antigen 2 (BST2) influences differentiation toward the osteoblasts lineage. BST2 mRNA expression in human alveolar-derived bone marrow stromal cells (hAD-BMSCs) increased during differentiation into osteoblasts. hAD-BMSCs differentiation into osteoblasts and the mRNA expression of the bone-specific markers alkaline phosphatase, collagen type α 1, bone sialoprotein, osteocalcin, and osterix were reduced by BST2 knockdown using siRNA. Furthermore, BST2 knockdown in hAD-BMSCs resulted in decreased RUNX2 mRNA and protein expression. We hypothesized that BST2 is involved in differentiation of into osteoblasts via the BMP2 signaling pathway. Accordingly, we evaluated the mRNA expression levels of BMP2, BMP receptors (BMPR1 and 2), and the downstream signaling molecules SMAD1, SMAD4, and p-SMAD1/5/8 in BST2 knockdown cells. BMP2 expression following the induction of differentiation was significantly lower in BST2 knockdown cells than in cells treated with a non-targeting control siRNA. Similar results were found for the knockdown of the BMP2 receptor- BMPR1A. We also identified significantly lower expression of SMAD1, SMAD4, and p-SMAD1/5/8 in the BST2 knockdown cells than control cells. Our data provide the first evidence that BST2 is involved in the osteogenic differentiation of bone marrow stromal cells via the regulation of the BMP2 signaling pathway.
Conflict of interest statement
Figures



Similar articles
-
Extracellular calcium promotes bone formation from bone marrow mesenchymal stem cells by amplifying the effects of BMP-2 on SMAD signalling.PLoS One. 2017 May 25;12(5):e0178158. doi: 10.1371/journal.pone.0178158. eCollection 2017. PLoS One. 2017. PMID: 28542453 Free PMC article.
-
Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells.Exp Mol Med. 2015 Jan 9;47(1):e128. doi: 10.1038/emm.2014.95. Exp Mol Med. 2015. PMID: 25572360 Free PMC article.
-
Age-related CXC chemokine receptor-4-deficiency impairs osteogenic differentiation potency of mouse bone marrow mesenchymal stromal stem cells.Int J Biochem Cell Biol. 2013 Aug;45(8):1813-20. doi: 10.1016/j.biocel.2013.05.034. Epub 2013 Jun 4. Int J Biochem Cell Biol. 2013. PMID: 23742988
-
[RESEARCH PROGRESS OF Hedgehog SIGNALING PATHWAY IN REGULATING BONE FORMATION AND OSTEOGENIC DIFFERENTIATION OF BONE MESENCHYMAL STEM CELLS].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016 Dec 8;30(12):1545-1550. doi: 10.7507/1002-1892.20160318. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016. PMID: 29786349 Review. Chinese.
-
[Regulation of osteogenic differentiation of mesenchimal stem sells of bone marrow].Ross Fiziol Zh Im I M Sechenova. 2013 Apr;99(4):417-32. Ross Fiziol Zh Im I M Sechenova. 2013. PMID: 23862383 Review. Russian.
Cited by
-
BST2 facilitates activation of hematopoietic stem cells through ERK signaling.Exp Hematol. 2024 Dec;140:104653. doi: 10.1016/j.exphem.2024.104653. Epub 2024 Oct 2. Exp Hematol. 2024. PMID: 39362577
-
Defective Proliferation and Osteogenic Potential with Altered Immunoregulatory phenotype of Native Bone marrow-Multipotential Stromal Cells in Atrophic Fracture Non-Union.Sci Rep. 2019 Nov 22;9(1):17340. doi: 10.1038/s41598-019-53927-3. Sci Rep. 2019. PMID: 31758052 Free PMC article.
-
Bioinformatics of Differentially Expressed Genes in Phorbol 12-Myristate 13-Acetate-Induced Megakaryocytic Differentiation of K562 Cells by Microarray Analysis.Int J Mol Sci. 2022 Apr 11;23(8):4221. doi: 10.3390/ijms23084221. Int J Mol Sci. 2022. PMID: 35457039 Free PMC article.
-
The enzymatic hydrolysates from deer sinew promote MC3T3-E1 cell proliferation and extracellular matrix synthesis by regulating multiple functional genes.BMC Complement Med Ther. 2021 Feb 10;21(1):59. doi: 10.1186/s12906-021-03240-2. BMC Complement Med Ther. 2021. PMID: 33568122 Free PMC article.
-
Single Nucleotide Polymorphisms in Runt-related Transcription Factor 2 and Bone Morphogenetic Protein 2 Impact on Their Maxillary and Mandibular Gene Expression in Different Craniofacial Patterns - A Comparative Study.Ann Maxillofac Surg. 2021 Jul-Dec;11(2):222-228. doi: 10.4103/ams.ams_40_21. Epub 2022 Feb 1. Ann Maxillofac Surg. 2021. PMID: 35265489 Free PMC article.
References
-
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2): 230–237. - PubMed
-
- Matsubara T, Suardita K, Ishii M, Sugiyama M, Igarashi A, Oda R, et al. Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 2005;20(3): 399–409. - PubMed
-
- Kagami H, Agata H, Inoue M, Asahina I, Tojo A, Yamashita N, et al. The use of bone marrow stromal cells (bone marrow-derived multipotent mesenchymal stromal cells) for alveolar bone tissue engineering: basic science to clinical translation. Tissue Eng Part B Rev. 2014;20(3): 229–232. 10.1089/ten.TEB.2013.0578 - DOI - PubMed
-
- Massagué J, Seoane J, Wotton D. Smad transcription factor. Genes Dev. 2005; 19:2783–2810. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

