Actin polymerization-dependent activation of Cas-L promotes immunological synapse stability
- PMID: 27359298
- PMCID: PMC5121033
- DOI: 10.1038/icb.2016.61
Actin polymerization-dependent activation of Cas-L promotes immunological synapse stability
Abstract
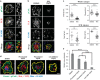
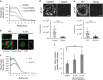
The immunological synapse formed between a T-cell and an antigen-presenting cell is important for cell-cell communication during T-cell-mediated immune responses. Immunological synapse formation begins with stimulation of the T-cell receptor (TCR). TCR microclusters are assembled and transported to the center of the immunological synapse in an actin polymerization-dependent process. However, the physical link between TCR and actin remains elusive. Here we show that lymphocyte-specific Crk-associated substrate (Cas-L), a member of a force sensing protein family, is required for transport of TCR microclusters and for establishing synapse stability. We found that Cas-L is phosphorylated at TCR microclusters in an actin polymerization-dependent fashion. Furthermore, Cas-L participates in a positive feedback loop leading to amplification of Ca2+ signaling, inside-out integrin activation, and actomyosin contraction. We propose a new role for Cas-L in T-cell activation as a mechanical transducer linking TCR microclusters to the underlying actin network and coordinating multiple actin-dependent structures in the immunological synapse. Our studies highlight the importance of mechanotransduction processes in T-cell-mediated immune responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
Mediating signaling response to actin-mediated forces: Cas-L is causal in the T-cell response to forces triggered by antigen presentation.Immunol Cell Biol. 2016 Nov;94(10):905-906. doi: 10.1038/icb.2016.79. Epub 2016 Sep 20. Immunol Cell Biol. 2016. PMID: 27645156 No abstract available.
References
-
- Crabtree GR. Contingent genetic regulatory events in T lymphocyte activation. Science 1989; 243: 355–361. - PubMed
-
- Weiss A. Molecular and genetic insights into T cell antigen receptor structure and function. Annu Rev Genet 1991; 25: 487–510. - PubMed
-
- Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell 1994; 76: 241–251. - PubMed
-
- Dustin ML, Miller JM, Ranganath S, Vignali DA, Viner NJ, Nelson CA et al TCR‐mediated adhesion of T cell hybridomas to planar bilayers containing purified MHC class II/peptide complexes and receptor shedding during detachment. J Immunol 1996; 157: 2014–2021. - PubMed
-
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three‐dimensional segregation of supramolecular activation clusters in T cells. Nature 1998; 395: 82–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

