Claudin-2-mediated cation and water transport share a common pore
- PMID: 27359349
- PMCID: PMC5201457
- DOI: 10.1111/apha.12742
Claudin-2-mediated cation and water transport share a common pore
Abstract
Aim: Claudin-2 is a tight junction protein typically located in 'leaky' epithelia exhibiting large paracellular permeabilities like small intestine and proximal kidney tubule. Former studies revealed that claudin-2 forms paracellular channels for small cations like sodium and potassium and also paracellular channels for water. This study analyses whether the diffusive transport of sodium and water occurs through a common pore of the claudin-2 channel.
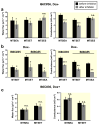
Methods: Wild-type claudin-2 and different claudin-2 mutants were expressed in MDCK I kidney tubule cells using an inducible system. Ion and water permeability and the effect of blocking reagents on both were investigated on different clones of the mutants.
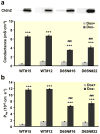
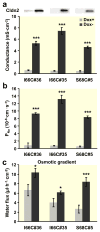
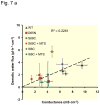
Results: Neutralization of a negatively charged cation interaction site in the pore with the mutation, D65N, decreased both sodium permeability and water permeability. Claudin-2 mutants (I66C and S68C) with substitution of the pore-lining amino acids with cysteine were used to test the effect of steric blocking of the claudin-2 pore by thiol-reactive reagents. Addition of thiol-reactive reagents to these mutants simultaneously decreased conductance and water permeability. Remarkably, all experimental perturbations caused parallel changes in ion conductance and water permeability, disproving different or independent passage pathways.
Conclusion: Our results indicate that claudin-2-mediated cation and water transport are frictionally coupled and share a common pore. This pore is lined and determined in permeability by amino acid residues of the first extracellular loop of claudin-2.
Keywords: claudin-2 pore; common pore for sodium and water; paracellular transport.
© 2016 Scandinavian Physiological Society. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Declaration The authors declare that they have no conflict of interest. The authors confirm that the material submitted is conform with Good Publishing Practice in Physiology 2013: Guidelines for Acta Physiologica (Persson PB, 2013).
Figures




References
-
- Agre P, Preston GM, Smith BL, Jung JS, Raina S, Moon C, Guggino WB, Nielsen S. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol. 1993;265:463–76. - PubMed
-
- Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–76. - PubMed
-
- Angelow S, El-Husseini R, Kanzawa SA, Yu AS. Renal localization and function of the tight junction protein, claudin-19. Am J Physiol Renal Physiol. 2007;293:166–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

