Allosteric coupling from G protein to the agonist-binding pocket in GPCRs
- PMID: 27362234
- PMCID: PMC5702553
- DOI: 10.1038/nature18324
Allosteric coupling from G protein to the agonist-binding pocket in GPCRs
Abstract
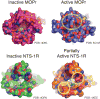
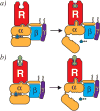
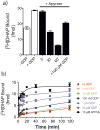
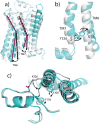
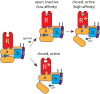
G-protein-coupled receptors (GPCRs) remain the primary conduit by which cells detect environmental stimuli and communicate with each other. Upon activation by extracellular agonists, these seven-transmembrane-domain-containing receptors interact with heterotrimeric G proteins to regulate downstream second messenger and/or protein kinase cascades. Crystallographic evidence from a prototypic GPCR, the β2-adrenergic receptor (β2AR), in complex with its cognate G protein, Gs, has provided a model for how agonist binding promotes conformational changes that propagate through the GPCR and into the nucleotide-binding pocket of the G protein α-subunit to catalyse GDP release, the key step required for GTP binding and activation of G proteins. The structure also offers hints about how G-protein binding may, in turn, allosterically influence ligand binding. Here we provide functional evidence that G-protein coupling to the β2AR stabilizes a ‘closed’ receptor conformation characterized by restricted access to and egress from the hormone-binding site. Surprisingly, the effects of G protein on the hormone-binding site can be observed in the absence of a bound agonist, where G-protein coupling driven by basal receptor activity impedes the association of agonists, partial agonists, antagonists and inverse agonists. The ability of bound ligands to dissociate from the receptor is also hindered, providing a structural explanation for the G-protein-mediated enhancement of agonist affinity, which has been observed for many GPCR–G-protein pairs. Our data also indicate that, in contrast to agonist binding alone, coupling of a G protein in the absence of an agonist stabilizes large structural changes in a GPCR. The effects of nucleotide-free G protein on ligand-binding kinetics are shared by other members of the superfamily of GPCRs, suggesting that a common mechanism may underlie G-protein-mediated enhancement of agonist affinity.
Figures
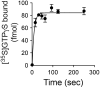

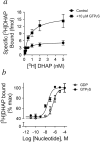

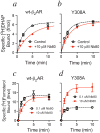

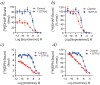



References
-
- Pierce KL, 1, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002 Sep;3(9):639–50. - PubMed
-
- Venter JC, et al. The sequence of the human genome. Science. 2001;291:1304–51. - PubMed
-
- Sprang SR. G protein mechanisms: insights from structural analysis. Annu Rev Biochem. 1997;66:639–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 GM106990/GM/NIGMS NIH HHS/United States
- P60DK-20572/DK/NIDDK NIH HHS/United States
- R01-GM083118/GM/NIGMS NIH HHS/United States
- R01-GM068603/GM/NIGMS NIH HHS/United States
- T32 GM007767/GM/NIGMS NIH HHS/United States
- T32GM007767/GM/NIGMS NIH HHS/United States
- R01 GM083118/GM/NIGMS NIH HHS/United States
- T32 GM008270/GM/NIGMS NIH HHS/United States
- P60 DK020572/DK/NIDDK NIH HHS/United States
- R01-NS28471/NS/NINDS NIH HHS/United States
- R01 NS028471/NS/NINDS NIH HHS/United States
- T32GM007315/GM/NIGMS NIH HHS/United States
- R01 GM068603/GM/NIGMS NIH HHS/United States
- R21 DA031418/DA/NIDA NIH HHS/United States
- T32GM008270/GM/NIGMS NIH HHS/United States
- U19-GM106990/GM/NIGMS NIH HHS/United States
- T32 GM007315/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

