A core viral protein binds host nucleosomes to sequester immune danger signals
- PMID: 27362237
- PMCID: PMC4950998
- DOI: 10.1038/nature18317
A core viral protein binds host nucleosomes to sequester immune danger signals
Abstract
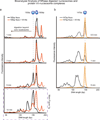
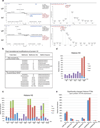
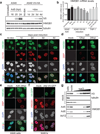
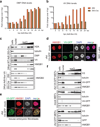
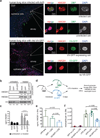
Viral proteins mimic host protein structure and function to redirect cellular processes and subvert innate defenses. Small basic proteins compact and regulate both viral and cellular DNA genomes. Nucleosomes are the repeating units of cellular chromatin and play an important part in innate immune responses. Viral-encoded core basic proteins compact viral genomes, but their impact on host chromatin structure and function remains unexplored. Adenoviruses encode a highly basic protein called protein VII that resembles cellular histones. Although protein VII binds viral DNA and is incorporated with viral genomes into virus particles, it is unknown whether protein VII affects cellular chromatin. Here we show that protein VII alters cellular chromatin, leading us to hypothesize that this has an impact on antiviral responses during adenovirus infection in human cells. We find that protein VII forms complexes with nucleosomes and limits DNA accessibility. We identified post-translational modifications on protein VII that are responsible for chromatin localization. Furthermore, proteomic analysis demonstrated that protein VII is sufficient to alter the protein composition of host chromatin. We found that protein VII is necessary and sufficient for retention in the chromatin of members of the high-mobility-group protein B family (HMGB1, HMGB2 and HMGB3). HMGB1 is actively released in response to inflammatory stimuli and functions as a danger signal to activate immune responses. We showed that protein VII can directly bind HMGB1 in vitro and further demonstrated that protein VII expression in mouse lungs is sufficient to decrease inflammation-induced HMGB1 content and neutrophil recruitment in the bronchoalveolar lavage fluid. Together, our in vitro and in vivo results show that protein VII sequesters HMGB1 and can prevent its release. This study uncovers a viral strategy in which nucleosome binding is exploited to control extracellular immune signaling.
Figures





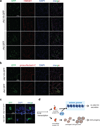
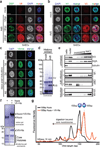
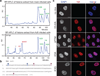
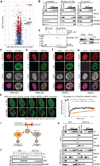
Comment in
-
Color Me Infected: Painting Cellular Chromatin with a Viral Histone Mimic.Trends Microbiol. 2016 Oct;24(10):774-776. doi: 10.1016/j.tim.2016.08.005. Epub 2016 Sep 1. Trends Microbiol. 2016. PMID: 27592243
References
-
- Elde NC, Malik HS. The evolutionary conundrum of pathogen mimicry. Nature Reviews Microbiology. 2009;7:787–797. - PubMed
-
- Smale ST, Tarakhovsky A, Natoli G. Chromatin contributions to the regulation of innate immunity. Annu. Rev. Immunol. 2014;32:489–511. - PubMed
-
- Lischwe MA, Sung MT. A histone-like protein from adenovirus chromatin. Nature. 1977;267:552–554. - PubMed
Methods References
-
- Kozarsky KF, Jooss K, Donahee M, Strauss JF, Wilson JM. Effective treatment of familial hypercholesterolaemia in the mouse model using adenovirus-mediated transfer of the VLDL receptor gene. Nat. Genet. 1996;13:54–62. - PubMed
-
- Reich NC, Sarnow P, Duprey E, Levine AJ. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology. 1983;128:480–484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 GM112414/GM/NIGMS NIH HHS/United States
- AI102577/AI/NIAID NIH HHS/United States
- R01 AI099479/AI/NIAID NIH HHS/United States
- T32 NS007180/NS/NINDS NIH HHS/United States
- R01 GM082989/GM/NIGMS NIH HHS/United States
- R01 CA122677/CA/NCI NIH HHS/United States
- R01 CA097093/CA/NCI NIH HHS/United States
- R21 AI102577/AI/NIAID NIH HHS/United States
- CA122677/CA/NCI NIH HHS/United States
- GM110174/GM/NIGMS NIH HHS/United States
- R01 AI118891/AI/NIAID NIH HHS/United States
- R01 GM110174/GM/NIGMS NIH HHS/United States
- GM082989/GM/NIGMS NIH HHS/United States
- AI118891/AI/NIAID NIH HHS/United States
- T32 CA115299/CA/NCI NIH HHS/United States
- CA097093/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

