Ovarian Grafts 10 Days after Xenotransplantation: Folliculogenesis and Recovery of Viable Oocytes
- PMID: 27362486
- PMCID: PMC4928796
- DOI: 10.1371/journal.pone.0158109
Ovarian Grafts 10 Days after Xenotransplantation: Folliculogenesis and Recovery of Viable Oocytes
Abstract
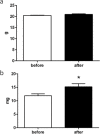
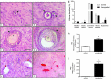
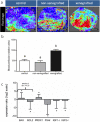
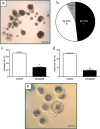
Ovarian xenotransplantation is a promising alternative to preserve fertility of oncologic patients. However, several functional aspects of this procedure remained to be addressed. The aim of this study was evaluate the feasibility of xenotransplantation as a strategy to maintain bovine ovarian grafts and produce oocytes. Adult ovarian cortical pieces were xenotransplanted to the dorsal subcutaneous of female NOD-SCID mice (n = 62). Grafts were recovered ten days after xenotransplantation. Host and graft weights; folliculogenesis progression; blood perfusion, relative gene expression and number of macrophage and neutrophil of xenografts; in vitro developmental competence of graft-derived oocytes were evaluated. Folliculogenesis was supported in the grafts, as indicated by the presence of primordial, primary, secondary, antral, and atretic follicles. The xenografts showed a greater volumetric density of atretic follicles and higher hyperemia and number of host-derived macrophage and neutrophil (P<0.05), when compared to non-grafted fragments. There was a higher blood perfusion under the back skin in the transplantation sites of host animals than in control and non-grafted (P<0.01). BAX and PRDX1 genes were up-regulated, while BCL2, FSHR, IGF1R and IGF2R were down-regulated, when compared to the control (P<0.01). Twenty seven oocytes were successfully harvested from grafts, and some of these oocytes were able to give rise to blastocysts after in vitro fertilization. However, cleavage and blastocyst rates of xenograft derived oocytes were lower than in control (P<0.01). Despite showing some functional modifications, the ovarian xenografts were able to support folliculogenesis and produce functional oocytes.
Conflict of interest statement
Figures
References
-
- Dittrich R, Maltaris T, Hoffmann I, Oppelt PG, Beckmann MW, Mueller A. Fertility preservation in cancer patients. Minerva Ginecol. 2010. 62; 63–80. - PubMed
-
- Kim SS, Soules MR, Battaglia DE. Follicular development, ovulation, and corpus luteum formation in cryopreserved human ovarian tissue after xenotransplantation. Fertil Steril. 2002. 78; 77–82. - PubMed
-
- von Schönfeldt V, Chandolia R, Kiesel L, Nieschlag E, Schlatt S, Sonntag B. Advanced follicle development in xenografted prepubertal ovarian tissue: the common marmoset as a nonhuman primate model for ovarian tissue transplantation. Fertil Steril. 2011. 95; 1428–34. 10.1016/j.fertnstert.2010.11.003 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

