Time Savings with Rituximab Subcutaneous Injection versus Rituximab Intravenous Infusion: A Time and Motion Study in Eight Countries
- PMID: 27362533
- PMCID: PMC4928781
- DOI: 10.1371/journal.pone.0157957
Time Savings with Rituximab Subcutaneous Injection versus Rituximab Intravenous Infusion: A Time and Motion Study in Eight Countries
Abstract
Background: Rituximab is a standard treatment for non-Hodgkin lymphoma. The SABRINA trial (NCT01200758) showed that a subcutaneous (SC) rituximab formulation did not compromise efficacy or safety compared with intravenous (IV) infusion. We aimed to quantify active healthcare professional (HCP) time and patient chair time for rituximab SC and IV, including potential time savings.
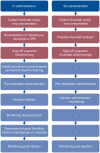
Methods: This non-interventional time and motion study was run in eight countries and 30 day oncology units. Rituximab SC data were collected alongside the MabCute trial (NCT01461928); IV data were collected per routine real-world practice. Trained observers recorded active HCP time for pre-specified tasks (stopwatch) and chair time (time of day). A random intercept model was used to analyze active HCP time (by task and for all tasks combined) in the treatment room and drug preparation area, drug administration duration, chair time and patient treatment room time by country and/or across countries. Active HCP and chair time were extrapolated to a patient's first year of treatment (11 rituximab sessions).
Results: Mean active HCP time was 35.0 and 23.7 minutes for IV and SC process, respectively (-32%, p <0.0001). By country, relative reduction in time was 27-58%. Absolute reduction in extrapolated active HCP time (first year of treatment) was 1.1-5.2 hours. Mean chair time was 262.1 minutes for IV, including 180.9 minutes infusion duration, vs. 67.3 minutes for SC, including 8.3 minutes SC injection administration (-74%, p <0.0001). By country, relative reduction was 53-91%. Absolute reduction in extrapolated chair time for the first year of treatment was 3.1-5.5 eight-hour days.
Conclusions: Compared with rituximab IV, rituximab SC was associated with reduced chair time and active HCP time. The latter could be invested in other activities, whereas the former may lead to more available appointments, reducing waiting lists and increasing the efficiency of day oncology units.
Trial registration: ClinicalTrials.gov NCT01200758.
Conflict of interest statement
Figures






References
-
- SEER (Surveillance, Epidemiology and End Results Program): Stat Fact Sheets: Non-Hodgkin Lymphoma. 2015. Available: http://seer.cancer.gov/statfacts/html/nhl.html.
-
- Marcus R, Hagenbeek A. The therapeutic use of rituximab in non-Hodgkin's lymphoma. Eur J Haematol. 2007;67(Suppl.):5–14. - PubMed
-
- European Medicines Agency: MabThera. 2009. Available: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici....
-
- Scott SD. Rituximab: a new therapeutic monoclonal antibody for non-Hodgkin’s lymphoma. Cancer Pract. 1998;6:195–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

