The contribution of arachidonate 15-lipoxygenase in tissue macrophages to adipose tissue remodeling
- PMID: 27362803
- PMCID: PMC5108340
- DOI: 10.1038/cddis.2016.190
The contribution of arachidonate 15-lipoxygenase in tissue macrophages to adipose tissue remodeling
Abstract
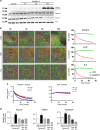
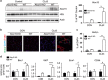
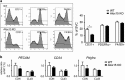
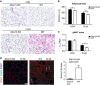
Cellular plasticity in adipose tissue involves adipocyte death, its clearance, and de novo adipogenesis, enabling homeostatic turnover and adaptation to metabolic challenges; however, mechanisms regulating these serial events are not fully understood. The present study investigated the roles of arachidonate 15-lipoxygenase (Alox15) in the clearance of dying adipocytes by adipose tissue macrophages. First, upregulation of Alox15 expression and apoptotic adipocyte death in gonadal white adipose tissue (gWAT) were characterized during adipose tissue remodeling induced by β3-adrenergic receptor stimulation. Next, an in vitro reconstruction of adipose tissue macrophages and apoptotic adipocytes recapitulated adipocyte clearance by macrophages and demonstrated that macrophages co-cultured with apoptotic adipocytes increased the expression of efferocytosis-related genes. Genetic deletion and pharmacological inhibition of Alox15 diminished the levels of adipocyte clearance by macrophages in a co-culture system. Gene expression profiling of macrophages isolated from gWAT of Alox15 knockout (KO) mice demonstrated distinct phenotypes, especially downregulation of genes involved in lipid uptake and metabolism compared to wild-type mice. Finally, in vivo β3-adrenergic stimulation in Alox15 KO mice failed to recruit crown-like structures, a macrophage network clearing dying adipocytes in gWAT. Consequently, in Alox15 KO mice, proliferation/differentiation of adipocyte progenitors and β3-adrenergic remodeling of gWAT were impaired compared to wild-type control mice. Collectively, our data established a pivotal role of Alox15 in the resolution of adipocyte death and in adipose tissue remodeling.
Figures



Similar articles
-
Adipogenic role of alternatively activated macrophages in β-adrenergic remodeling of white adipose tissue.Am J Physiol Regul Integr Comp Physiol. 2016 Jan 1;310(1):R55-65. doi: 10.1152/ajpregu.00355.2015. Epub 2015 Nov 4. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26538237 Free PMC article.
-
MicroRNA-10a-5p regulates macrophage polarization and promotes therapeutic adipose tissue remodeling.Mol Metab. 2019 Nov;29:86-98. doi: 10.1016/j.molmet.2019.08.015. Epub 2019 Aug 27. Mol Metab. 2019. PMID: 31668395 Free PMC article.
-
Identification of an adipogenic niche for adipose tissue remodeling and restoration.Cell Metab. 2013 Sep 3;18(3):355-67. doi: 10.1016/j.cmet.2013.08.003. Cell Metab. 2013. PMID: 24011071 Free PMC article.
-
How obesity affects adipocyte turnover.Trends Endocrinol Metab. 2025 Feb;36(2):147-160. doi: 10.1016/j.tem.2024.07.004. Epub 2024 Aug 1. Trends Endocrinol Metab. 2025. PMID: 39095230 Review.
-
Adipose tissue plasticity and the pleiotropic roles of BMP signaling.J Biol Chem. 2021 Jan-Jun;296:100678. doi: 10.1016/j.jbc.2021.100678. Epub 2021 Apr 17. J Biol Chem. 2021. PMID: 33872596 Free PMC article. Review.
Cited by
-
Bta-miR-33a affects gene expression and lipid levels in Chinese Holstein mammary epithelial cells.Arch Anim Breed. 2022 Oct 10;65(4):357-370. doi: 10.5194/aab-65-357-2022. eCollection 2022. Arch Anim Breed. 2022. PMID: 36304442 Free PMC article.
-
Anti-inflammatory role of 15-lipoxygenase contributes to the maintenance of skin integrity in mice.Sci Rep. 2018 Jun 11;8(1):8856. doi: 10.1038/s41598-018-27221-7. Sci Rep. 2018. PMID: 29891910 Free PMC article.
-
Global transcriptome analysis identifies weight regain-induced activation of adaptive immune responses in white adipose tissue of mice.Int J Obes (Lond). 2018 Apr;42(4):755-764. doi: 10.1038/ijo.2017.297. Epub 2017 Dec 7. Int J Obes (Lond). 2018. PMID: 29762555 Free PMC article.
-
Passive Transfer of Vaccine-Elicited Antibodies Protects against SIV in Rhesus Macaques.Cell. 2020 Oct 1;183(1):185-196.e14. doi: 10.1016/j.cell.2020.08.033. Cell. 2020. PMID: 33007262 Free PMC article.
-
A Novel Function for 15-Lipoxygenases in Cholesterol Homeostasis and CCL17 Production in Human Macrophages.Front Immunol. 2018 Aug 24;9:1906. doi: 10.3389/fimmu.2018.01906. eCollection 2018. Front Immunol. 2018. PMID: 30197642 Free PMC article.
References
-
- Gesta S, Tseng YH, Kahn CR.. Developmental origin of fat: tracking obesity to its source. Cell 2007; 131: 242–256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

