Cell-free DNA testing of an extended range of chromosomal anomalies: clinical experience with 6,388 consecutive cases
- PMID: 27362910
- PMCID: PMC5303761
- DOI: 10.1038/gim.2016.72
Cell-free DNA testing of an extended range of chromosomal anomalies: clinical experience with 6,388 consecutive cases
Abstract
Purpose: Cell-free DNA (cfDNA) testing for fetal aneuploidies was broadly implemented for common trisomies and sex-chromosome anomalies (SCAs). However, such an approach identifies only 75 to 85% of clinically relevant aneuploidies.
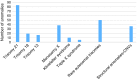
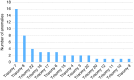
Methods: We present a consecutive series of 6,388 cases, thus uncovering a broader array of aneuploidies, including the rare autosomal trisomies (RATs) and the maternally inherited deletion and duplication copy-number variations (CNVs), with complete and stratified follow-up by amniocentesis. Combined measurements of z-scores and the fetal fraction, in conjunction with fetal cfDNA enrichment, were used to stratify the likelihood of true and false results.
Results: We obtained an incremental diagnostic yield of 50%; RATs and CNVs were found to be significant causes of fetal pathology. Scrutinizing z-scores and the fetal fraction made it possible to distinguish the sources of false-negative results; predict the likelihood of false-positive results for major trisomies and SCAs; classify maternal mosaic SCAs and CNVs, preventing false-positive results; and robustly identify maternally inherited CNVs and detect recurrent genomic disorders as a standardized function of the fetal fraction.
Conclusion: With the clinical pertinence of this broader detection scheme confirmed, we offer recommendations for its implementation.Genet Med 19 2, 169-175.
Figures

References
-
- Gil MM, Quezada MS, Revello R, Akolekar R, Nicolaides KH. Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol 2015;45:249–266. - PubMed
-
- Zhang H, Gao Y, Jiang F, et al. Non-invasive prenatal testing for trisomies 21, 18 and 13: clinical experience from 146,958 pregnancies. Ultrasound Obstet Gynecol 2015;45:530–538. - PubMed
-
- Bianchi DW, Parsa S, Bhatt S, et al. Fetal sex chromosome testing by maternal plasma DNA sequencing: clinical laboratory experience and biology. Obstet Gynecol 2015;125:375–382. - PubMed
-
- Davis C, Cuckle H, Yaron Y. Screening for Down syndrome–incidental diagnosis of other aneuploidies. Prenat Diagn 2014;34:1044–1048. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

