Control of Insulin Secretion by Production of Reactive Oxygen Species: Study Performed in Pancreatic Islets from Fed and 48-Hour Fasted Wistar Rats
- PMID: 27362938
- PMCID: PMC4928816
- DOI: 10.1371/journal.pone.0158166
Control of Insulin Secretion by Production of Reactive Oxygen Species: Study Performed in Pancreatic Islets from Fed and 48-Hour Fasted Wistar Rats
Abstract
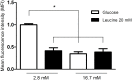
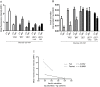
Mitochondria and NADPH oxidase are important sources of reactive oxygen species in particular the superoxide radical (ROS) in pancreatic islets. These molecules derived from molecular oxygen are involved in pancreatic β-cells signaling and control of insulin secretion. We examined the involvement of ROS produced through NADPH oxidase in the leucine- and/or glucose-induced insulin secretion by pancreatic islets from fed or 48-hour fasted rats. Glucose-stimulated insulin secretion (GSIS) in isolated islets was evaluated at low (2.8 mM) or high (16.7 mM) glucose concentrations in the presence or absence of leucine (20 mM) and/or NADPH oxidase inhibitors (VAS2870-20 μM or diphenylene iodonium-DPI-5 μM). ROS production was determined in islets treated with dihydroethidium (DHE) or MitoSOX Red reagent for 20 min and dispersed for fluorescence measurement by flow cytometry. NADPH content variation was examined in INS-1E cells (an insulin secreting cell line) after incubation in the presence of glucose (2.8 or 16.7 mM) and leucine (20 mM). At 2.8 mM glucose, VAS2870 and DPI reduced net ROS production (by 30%) and increased GSIS (by 70%) in a negative correlation manner (r = -0.93). At 16.7 mM glucose or 20 mM leucine, both NADPH oxidase inhibitors did not alter insulin secretion neither net ROS production. Pentose phosphate pathway inhibition by treatment with DHEA (75 μM) at low glucose led to an increase in net ROS production in pancreatic islets from fed rats (by 40%) and induced a marked increase (by 144%) in islets from 48-hour fasted rats. The NADPH/NADP+ ratio was increased when INS-1E cells were exposed to high glucose (by 4.3-fold) or leucine (by 3-fold). In conclusion, increased ROS production through NADPH oxidase prevents the occurrence of hypoglycemia in fasting conditions, however, in the presence of high glucose or high leucine levels, the increased production of NADPH and the consequent enhancement of the activity of the antioxidant defenses mitigate the excess of ROS production and allow the secretory process of insulin to take place.
Conflict of interest statement
Figures






References
-
- Oliveira HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR. Pancreatic beta-cells express phagocyte-like NAD(P)H oxidase. Diabetes. 2003;52(6):1457–63. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

