The posterior HOXD locus: Its contribution to phenotype and malignancy of Ewing sarcoma
- PMID: 27363011
- PMCID: PMC5173095
- DOI: 10.18632/oncotarget.9702
The posterior HOXD locus: Its contribution to phenotype and malignancy of Ewing sarcoma
Abstract
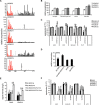
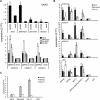
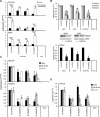
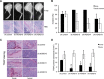
Microarray analysis revealed genes of the posterior HOXD locus normally involved in bone formation to be over-expressed in primary Ewing sarcoma (ES). The expression of posterior HOXD genes was not influenced via ES pathognomonic EWS/ETS translocations. However, knock down of the dickkopf WNT signaling pathway inhibitor 2 (DKK2) resulted in a significant suppression of HOXD10, HOXD11 and HOXD13 while over-expression of DKK2 and stimulation with factors of the WNT signaling pathway such as WNT3a, WNT5a or WNT11 increased their expression. RNA interference demonstrated that individual HOXD genes promoted chondrogenic differentiation potential, and enhanced expression of the bone-associated gene RUNX2. Furthermore, HOXD genes increased the level of the osteoblast- and osteoclast-specific genes, osteocalcin (BGLAP) and platelet-derived growth factor beta polypeptide (PDGFB), and may further regulate endochondral bone development via induction of parathyroid hormone-like hormone (PTHLH). Additionally, HOXD11 and HOXD13 promoted contact independent growth of ES, while in vitro invasiveness of ES lines was enhanced by all 3 HOXD genes investigated and seemed mediated via matrix metallopeptidase 1 (MMP1). Consequently, knock down of HOXD11 or HOXD13 significantly suppressed lung metastasis in a xeno-transplant model in immune deficient mice, providing overall evidence that posterior HOXD genes promote clonogenicity and metastatic potential of ES.
Keywords: Ewing sarcoma; HOXD; WNT signaling; endochondral development; metastasis.
Conflict of interest statement
The authors declare no conflict of interest or financial interests.
Figures

References
-
- Mackintosh C, Madoz-Gurpide J, Ordonez JL, Osuna D, Herrero-Martin D. The molecular pathogenesis of Ewing's sarcoma. Cancer biology & therapy. 2010;9:655–667. - PubMed
-
- Ewing J. Classics in oncology. Diffuse endothelioma of bone. James Ewing. Proceedings of the New York Pathological Society, 1921. CA Cancer J Clin. 1972;22:95–98. - PubMed
-
- Schmidt D, Harms D, Burdach S. Malignant peripheral neuroectodermal tumours of childhood and adolescence. Virchows Arch A Pathol Anat Histopathol. 1985;406:351–365. - PubMed
-
- Hauer K, Calzada-Wack J, Steiger K, Grunewald TG, Baumhoer D, Plehm S, Buch T, Prazeres da Costa O, Esposito I, Burdach S, Richter GH. DKK2 mediates osteolysis, invasiveness, and metastatic spread in Ewing sarcoma. Cancer Res. 2013;73:967–977. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

