Regression of experimental NIS-expressing breast cancer brain metastases in response to radioiodide/gemcitabine dual therapy
- PMID: 27363025
- PMCID: PMC5342383
- DOI: 10.18632/oncotarget.10238
Regression of experimental NIS-expressing breast cancer brain metastases in response to radioiodide/gemcitabine dual therapy
Abstract
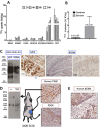
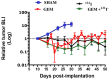
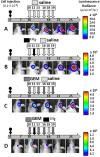
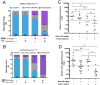
Treating breast cancer brain metastases (BCBMs) is challenging. Na+/I- symporter (NIS) expression in BCBMs would permit their selective targeting with radioiodide (131I-). We show impressive enhancement of tumor response by combining131I- with gemcitabine (GEM), a cytotoxic radiosensitizer. Nude mice mammary fat-pad (MFP) tumors and BCBMs were generated with braintropic MDA-MB-231Br cells transduced with bicistronically-linked NIS and firefly luciferase cDNAs. Response was monitored in vivo via bioluminescent imaging and NIS tumor expression.131I-/GEM therapy inhibited MFP tumor growth more effectively than either agent alone. BCBMs were treated with: high or low-dose GEM (58 or 14.5 mg/Kg×4); 131I- (1mCi or 2×0.5 mCi 7 days apart); and 131I-/GEM therapy. By post-injection day (PID) 25, 82-86% of controls and 78-83% of 131I--treated BCBM grew, whereas 17% low-dose and 36% high-dose GEM regressed. The latter tumors were smaller than the controls with comparable NIS expression (~20% of cells). High and low-dose 131I-/ GEM combinations caused 89% and 57% tumor regression, respectively. High-dose GEM/131I- delayed tumor growth: tumors increased 5-fold in size by PID45 (controls by PID18). Although fewer than 25% of cells expressed NIS, GEM/131I- caused dramatic tumor regression in NIS-transduced BCBMs. This effect was synergistic, and supports the hypothesis that GEM radiosensitizes cells to 131I-.
Keywords: breast cancer brain metastases (BCBMs); radioiodide therapy; sodium/iodide symporter (NIS).
Conflict of interest statement
None of the authors have any conflicts of interest to disclose with respect to the research described herein.
Figures


Similar articles
-
In vivo radioiodide imaging and treatment of breast cancer xenografts after MUC1-driven expression of the sodium iodide symporter.Clin Cancer Res. 2005 Feb 15;11(4):1483-9. doi: 10.1158/1078-0432.CCR-04-1636. Clin Cancer Res. 2005. PMID: 15746050
-
Hypoxia-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated sodium iodide symporter gene delivery.Oncotarget. 2016 Aug 23;7(34):54795-54810. doi: 10.18632/oncotarget.10758. Oncotarget. 2016. PMID: 27458162 Free PMC article.
-
Adenovirus-mediated and targeted expression of the sodium-iodide symporter permits in vivo radioiodide imaging and therapy of pancreatic tumors.Hum Gene Ther. 2006 Jun;17(6):661-8. doi: 10.1089/hum.2006.17.661. Hum Gene Ther. 2006. PMID: 16776574
-
A transporter gene (sodium iodide symporter) for dual purposes in gene therapy: imaging and therapy.Curr Gene Ther. 2002 Dec;2(4):393-402. doi: 10.2174/1566523023347599. Curr Gene Ther. 2002. PMID: 12477251 Review.
-
The sodium iodide symporter (NIS): regulation and approaches to targeting for cancer therapeutics.Pharmacol Ther. 2012 Sep;135(3):355-70. doi: 10.1016/j.pharmthera.2012.06.007. Epub 2012 Jun 29. Pharmacol Ther. 2012. PMID: 22750642 Free PMC article. Review.
Cited by
-
Expression of pendrin and NIS iodide transporters in human breast tumor and peri-tumoral tissue.Arch Med Sci. 2019 Nov 25;18(4):1041-1050. doi: 10.5114/aoms.2019.89980. eCollection 2022. Arch Med Sci. 2019. PMID: 35832691 Free PMC article.
-
Enhanced noninvasive imaging of oncology models using the NIS reporter gene and bioluminescence imaging.Cancer Gene Ther. 2020 Apr;27(3-4):179-188. doi: 10.1038/s41417-019-0081-2. Epub 2019 Jan 24. Cancer Gene Ther. 2020. PMID: 30674994 Free PMC article.
References
-
- Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, Hall WA, Hynynen K, Senter PD, Peereboom DM, Neuwelt EA. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25:2295–305. - PubMed
-
- Wapnir IL, van de Rijn M, Nowels K, Amenta PS, Walton K, Montgomery K, Greco RS, Dohan O, Carrasco N. Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J Clin Endocrinol Metab. 2003;88:1880–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

