Concerted action of IFN-α and IFN-λ induces local NK cell immunity and halts cancer growth
- PMID: 27363032
- PMCID: PMC5226505
- DOI: 10.18632/oncotarget.10272
Concerted action of IFN-α and IFN-λ induces local NK cell immunity and halts cancer growth
Abstract
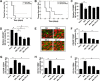
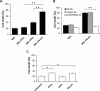
Hepatocellular carcinoma (HCC) is the most prevalent type of liver cancer. No significant improvement has been reported with currently available systemic therapies. IFN-α has been tested in both clinic and animal models and only moderate benefits have been observed. In animal models, similar modest antitumor efficacy has also been reported for IFN-λ, a new type of IFN that acts through its own receptor complex. In the present study, the antitumor efficacy of the combination of IFN-α and IFN-λ was tested in the BNL mouse hepatoma model. This study was accomplished by using either engineered tumor cells (IFN-α/IFN-λ gene therapy) or by directly injecting tumor-bearing mice with IFN-α/IFN-λ. Both approaches demonstrated that IFN-α/IFN-λ combination therapy was more efficacious than IFN monotherapy based on either IFN-α or IFN-λ. In complement to tumor surgery, IFN-α/IFN-λ combination induced complete tumor remission. Highest antitumor efficacy has been obtained following local administration of IFN-α/IFN-λ combination at the tumor site that was associated with strong NK cells tumor infiltration. This supports the use of IFN-α/IFN-λ combination as a new cancer immunotherapy for stimulating antitumor response after cancer surgery.
Keywords: HCV; IFN therapy; IFN-α/IFN-λ combination; hepatocellular carcinoma; tumor immunity.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures


References
-
- Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Seminars in liver disease. 2010;30:3–16. - PubMed
-
- Melloul E, Lesurtel M, Carr BI, Clavien PA. Developments in liver transplantation for hepatocellular carcinoma. Seminars in oncology. 2012;39:510–521. - PubMed
-
- Shimomura S, Ikeda N, Saito M, Ishii A, Takashima T, Sakai Y, Yoshikawa S, Aizawa N, Tanaka H, Iwata Y, Enomoto H, Imanishi H, Yamamoto T, et al. Long-term interferon therapy after radiofrequency ablation is effective in treating patients with HCV-associated hepatocellular carcinoma. Hepatol Int. 2010;5:559–566. - PMC - PubMed
-
- Lin SM, Yu ML, Lee CM, Chien RN, Sheen IS, Chu CM, Liaw YF. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol. 2007;46:45–52. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

