ITGAV and ITGA5 diversely regulate proliferation and adipogenic differentiation of human adipose derived stem cells
- PMID: 27363302
- PMCID: PMC4929468
- DOI: 10.1038/srep28889
ITGAV and ITGA5 diversely regulate proliferation and adipogenic differentiation of human adipose derived stem cells
Abstract
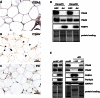
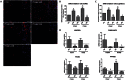
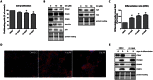
The fate of human adipose tissue stem cells (ASCs) is largely determined by biochemical and mechanical cues from the extracellular matrix (ECM), which are sensed and transmitted by integrins. It is well known that specific ECM constituents influence ASC proliferation and differentiation. Nevertheless, knowledge on how individual integrins regulate distinct processes is still limited. We performed gene profiling of 18 alpha integrins in sorted ASCs and adipocytes, identifying downregulations of RGD-motif binding integrins integrin-alpha-V (ITGAV) and integrin-alpha-5 (ITGA5), upregulation of laminin binding and leukocyte-specific integrins and individual regulations of collagen and LDV-receptors in differentiated adipocytes in-vivo. Gene function analyses in in-vitro cultured ASCs unraveled differential functions of ITGA5 and ITGAV. Knockdown of ITGAV, but not ITGA5 reduced proliferation, caused p21(Cip1) induction, repression of survivin and specific regulation of Hippo pathway mediator TAZ. Gene knockdown of both integrins promoted adipogenic differentiation, while transgenic expression impaired adipogenesis. Inhibition of ITGAV using cilengitide resulted in a similar phenotype, mimicking loss of pan-ITGAV expression using RNAi. Herein we show ASC specific integrin expression patterns and demonstrate distinct regulating roles of both integrins in human ASCs and adipocyte physiology suggesting a negative impact of RDG-motif signaling on adipogenic differentiation of ASCs via ITGA5 and ITGAV.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

