Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition
- PMID: 27363410
- PMCID: PMC5001499
- DOI: 10.1093/humupd/dmw022
Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition
Abstract
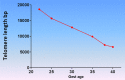
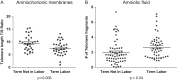
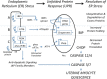
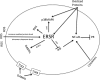
The signals and mechanisms that synchronize the timing of human parturition remain a mystery and a better understanding of these processes is essential to avert adverse pregnancy outcomes. Although our insights into human labor initiation have been informed by studies in animal models, the timing of parturition relative to fetal maturation varies among viviparous species, indicative of phylogenetically different clocks and alarms; but what is clear is that important common pathways must converge to control the birth process. For example, in all species, parturition involves the transition of the myometrium from a relaxed to a highly excitable state, where the muscle rhythmically and forcefully contracts, softening the cervical extracellular matrix to allow distensibility and dilatation and thus a shearing of the fetal membranes to facilitate their rupture. We review a number of theories promulgated to explain how a variety of different timing mechanisms, including fetal membrane cell senescence, circadian endocrine clocks, and inflammatory and mechanical factors, are coordinated as initiators and effectors of parturition. Many of these factors have been independently described with a focus on specific tissue compartments.In this review, we put forth the core hypothesis that fetal membrane (amnion and chorion) senescence is the initiator of a coordinated, redundant signal cascade leading to parturition. Whether modified by oxidative stress or other factors, this process constitutes a counting device, i.e. a clock, that measures maturation of the fetal organ systems and the production of hormones and other soluble mediators (including alarmins) and that promotes inflammation and orchestrates an immune cascade to propagate signals across different uterine compartments. This mechanism in turn sensitizes decidual responsiveness and eventually promotes functional progesterone withdrawal in the myometrium, leading to increased myometrial cell contraction and the triggering of parturition. Linkage of these processes allows convergence and integration of the gestational clocks and alarms, prompting a timely and safe birth. In summary, we provide a comprehensive synthesis of the mediators that contribute to the timing of human labor. Integrating these concepts will provide a better understanding of human parturition and ultimately improve pregnancy outcomes.
Keywords: NK cells; aging; corticosteroids; endoplasmic reticulum; labor and delivery; oxidative stress; p38MAPK; progesterone receptors; senescence; unfolded proteins.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

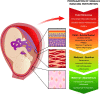
References
-
- Abdel-Hafiz H, Takimoto GS, Tung L, Horwitz KB. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J Biol Chem 2002;277:33950–33956. - PubMed
-
- ACOG Committee Opinion No 579: Definition of term pregnancy. Obstet Gynecol 2013;122:139–1140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

