A Complex Interaction Between Reduced Reelin Expression and Prenatal Organophosphate Exposure Alters Neuronal Cell Morphology
- PMID: 27364165
- PMCID: PMC4962342
- DOI: 10.1177/1759091416656253
A Complex Interaction Between Reduced Reelin Expression and Prenatal Organophosphate Exposure Alters Neuronal Cell Morphology
Abstract
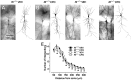
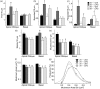
Genetic and environmental factors are both likely to contribute to neurodevelopmental disorders including schizophrenia, autism spectrum disorders, and major depressive disorders. Prior studies from our laboratory and others have demonstrated that the combinatorial effect of two factors-reduced expression of reelin protein and prenatal exposure to the organophosphate pesticide chlorpyrifos oxon-gives rise to acute biochemical effects and to morphological and behavioral phenotypes in adolescent and young adult mice. In the current study, we examine the consequences of these factors on reelin protein expression and neuronal cell morphology in adult mice. While the cell populations that express reelin in the adult brain appear unchanged in location and distribution, the levels of full length and cleaved reelin protein show persistent reductions following prenatal exposure to chlorpyrifos oxon. Cell positioning and organization in the hippocampus and cerebellum are largely normal in animals with either reduced reelin expression or prenatal exposure to chlorpyrifos oxon, but cellular complexity and dendritic spine organization is altered, with a skewed distribution of immature dendritic spines in adult animals. Paradoxically, combinatorial exposure to both factors appears to generate a rescue of the dendritic spine phenotypes, similar to the mitigation of behavioral and morphological changes observed in our prior study. Together, our observations support an interaction between reelin expression and chlorpyrifos oxon exposure that is not simply additive, suggesting a complex interplay between genetic and environmental factors in regulating brain morphology.
Keywords: cerebellum; cortex; dendrite; hippocampus; mouse model; pesticide.
© The Author(s) 2016.
Figures




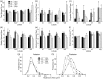
References
-
- Abadesco A., Cilluffo M., Yvone G., Carpenter E., Howell B., & Phelps P. (2014) Novel Disabled-1 expressing neurons identified in adult brain and spinal cord. European Journal of Neuroscience 39: 579–592. - PubMed
-
- Anderson S. A., Marin O., Horn C., Jennings K., Rubenstein J. L. R. (2001) Distinct cortical migration from the medial and lateral ganglionic eminences. Development 128: 353–363. - PubMed
-
- Biamonte F., Assenzab G., Marinoa R., D’Amelioc M., Panteria R., Carusod D., Keller F. (2009) Interactions between neuroactive steroids and reelin haploinsufficiency in Purkinje cell survival. Neurobiology of Disease 36: 103–115. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
