Phosphatidic Acid Sequesters Sec18p from cis-SNARE Complexes to Inhibit Priming
- PMID: 27364524
- PMCID: PMC5023487
- DOI: 10.1111/tra.12423
Phosphatidic Acid Sequesters Sec18p from cis-SNARE Complexes to Inhibit Priming
Abstract
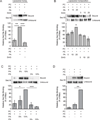
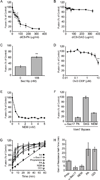
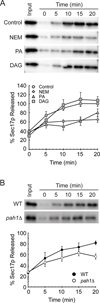
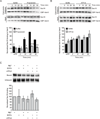
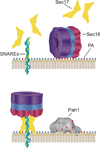
Yeast vacuole fusion requires the activation of cis-SNARE complexes through priming carried out by Sec18p/N-ethylmaleimide sensitive factor and Sec17p/α-SNAP. The association of Sec18p with vacuolar cis-SNAREs is regulated in part by phosphatidic acid (PA) phosphatase production of diacylglycerol (DAG). Inhibition of PA phosphatase activity blocks the transfer of membrane-associated Sec18p to SNAREs. Thus, we hypothesized that Sec18p associates with PA-rich membrane microdomains before transferring to cis-SNARE complexes upon PA phosphatase activity. Here, we examined the direct binding of Sec18p to liposomes containing PA or DAG. We found that Sec18p preferentially bound to liposomes containing PA compared with those containing DAG by approximately fivefold. Additionally, using a specific PA-binding domain blocked Sec18p binding to PA-liposomes and displaced endogenous Sec18p from isolated vacuoles. Moreover, the direct addition of excess PA blocked the priming activity of isolated vacuoles in a manner similar to chemically inhibiting PA phosphatase activity. These data suggest that the conversion of PA to DAG facilitates the recruitment of Sec18p to cis-SNAREs. Purified vacuoles from yeast lacking the PA phosphatase Pah1p showed reduced Sec18p association with cis-SNAREs and complementation with plasmid-encoded PAH1 or recombinant Pah1p restored the interaction. Taken together, this demonstrates that regulating PA concentrations by Pah1p activity controls SNARE priming by Sec18p.
Keywords: Pah1p; SNARE; Sec17; diacylglycerol; fusion; lipin; phosphatidic acid; priming.
© 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures


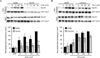
References
-
- Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu. Rev. Biochem. 1999:68863–68911. - PubMed
-
- Jahn R, Lang T, Südhof TC. Membrane fusion. Cell. 2003;112(4):519–533. - PubMed
-
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85(1):83–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

