Rapid Reprogramming of Primary Human Astrocytes into Potent Tumor-Initiating Cells with Defined Genetic Factors
- PMID: 27364552
- PMCID: PMC5082736
- DOI: 10.1158/0008-5472.CAN-16-0171
Rapid Reprogramming of Primary Human Astrocytes into Potent Tumor-Initiating Cells with Defined Genetic Factors
Abstract
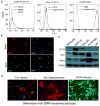
Cancer stem-like cells (CSC) are thought to drive brain cancer, but their cellular and molecular origins remain uncertain. Here, we report the successful generation of induced CSC (iCSC) from primary human astrocytes through the expression of defined genetic factors. Combined transduction of four factors, Myc, Oct-4, p53DD, and Ras, induced efficient transformation of primary human astrocytes into malignant cells with powerful tumor-initiating capabilities. Notably, transplantation of 100 transduced cells into nude mice was sufficient for tumor formation. The cells showed unlimited self-renewal ability with robust telomerase activities. In addition, they expressed typical glioma stem-like cell markers, such as CD133, CD15, and CD90. Moreover, these cells could form spheres in culture and differentiate into neuron-like, astrocyte-like, and oligodendrocyte-like cells. Finally, they also displayed resistance to the widely used brain cancer drug temozolomide. These iCSCs could provide important tools for studies of glioma biology and therapeutics development. Cancer Res; 76(17); 5143-50. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
The authors declare no financial interest in the work described in this study.
Figures



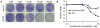
References
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

