Early goal-directed therapy in severe sepsis and septic shock: insights and comparisons to ProCESS, ProMISe, and ARISE
- PMID: 27364620
- PMCID: PMC4929762
- DOI: 10.1186/s13054-016-1288-3
Early goal-directed therapy in severe sepsis and septic shock: insights and comparisons to ProCESS, ProMISe, and ARISE
Abstract
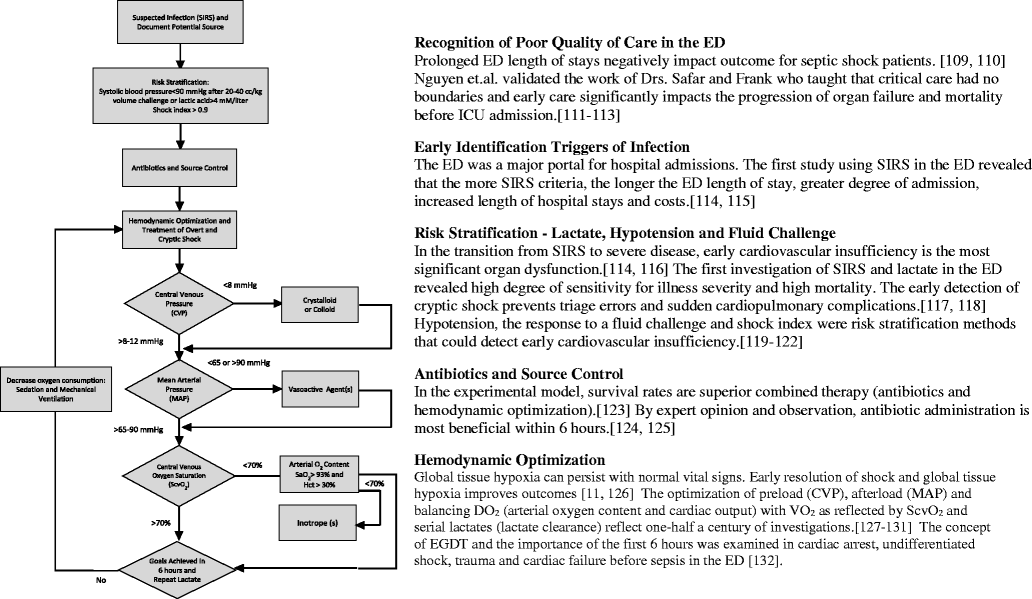
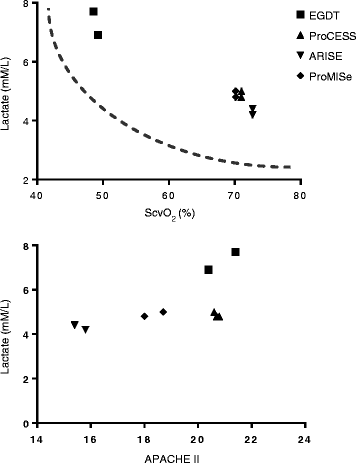
Prior to 2001 there was no standard for early management of severe sepsis and septic shock in the emergency department. In the presence of standard or usual care, the prevailing mortality was over 40-50 %. In response, a systems-based approach, similar to that in acute myocardial infarction, stroke and trauma, called early goal-directed therapy was compared to standard care and this clinical trial resulted in a significant mortality reduction. Since the publication of that trial, similar outcome benefits have been reported in over 70 observational and randomized controlled studies comprising over 70,000 patients. As a result, early goal-directed therapy was largely incorporated into the first 6 hours of sepsis management (resuscitation bundle) adopted by the Surviving Sepsis Campaign and disseminated internationally as the standard of care for early sepsis management. Recently a trio of trials (ProCESS, ARISE, and ProMISe), while reporting an all-time low sepsis mortality, question the continued need for all of the elements of early goal-directed therapy or the need for protocolized care for patients with severe and septic shock. A review of the early hemodynamic pathogenesis, historical development, and definition of early goal-directed therapy, comparing trial conduction methodology and the changing landscape of sepsis mortality, are essential for an appropriate interpretation of these trials and their conclusions.
Figures
Comment in
-
Work of breathing, not dysoxia, as the cause of low central venous blood O2 saturation in sepsis.Crit Care. 2016 Sep 19;20:291. doi: 10.1186/s13054-016-1476-1. Crit Care. 2016. PMID: 27640526 Free PMC article. No abstract available.
References
-
- Rosario AL, Park M, Brunialti MK, Mendes M, Rapozo M, Fernandes D, et al. SvO(2)-guided resuscitation for experimental septic shock: effects of fluid infusion and dobutamine on hemodynamics, inflammatory response, and cardiovascular oxidative stress. Shock. 2011;36(6):604–12. doi: 10.1097/SHK.0b013e3182336aa4. - DOI - PubMed
-
- Practice parameters for hemodynamic support of sepsis in adult patients in sepsis. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1999;27(3):639–60. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

